Lesson 1 of this chapter focused on the meaning of temperature and heat. Emphasis was given to the development of a particle model of matter that is capable of explaining the macroscopic observations. Efforts have been made to develop solid conceptual understandings of the topic in the absence of mathematical formulas. We learned that heat flows from one object to another (between the system and the surroundings) when a temperature difference exists between system and surroundings. Now in this unit we will investigate the topic of measuring the quantity of heat that is transferred between the system and the surroundings. This lesson is devoted to calorimetry - the science associated with determining the changes in energy of a system by measuring the heat exchanged with the surroundings. Before we can understand the mathematics of calorimetry, we should answer a critical question that was at least in part addressed in Lesson 1. The question is: what does heat do? When heat is lost or gained by an object, what does it do?
 For some students, the very question what does heat do? is confusing. Think about the question a moment. Does the question (not just the answer) confuse you? Confusion over the question is sometimes caused by misconceptions about what heat is. The reason for the lengthy discussions in Lesson 1 was to provide a solid conceptual foundation for understanding the mathematics of Lesson 2. If the question is confusing, you might want to review Lesson 1 or at least review the discussion pertaining to What is Heat? In Lesson 1, it was emphasized that heat is not something that is contained in an object. Objects do not contain heat. Objects, which are made of atoms, molecules and ions, contain energy. Heat is the transfer of energy from an object to its surroundings or to an object from its surroundings. So the question being asked on this page is what does this heat do to the object and to the surroundings when it is transferred? Like many questions in physics, it is a simple answer with deep meaning. Simple answers with deep meaning always exercise the brain. So put on your thinking cap and let's get to the answer.
For some students, the very question what does heat do? is confusing. Think about the question a moment. Does the question (not just the answer) confuse you? Confusion over the question is sometimes caused by misconceptions about what heat is. The reason for the lengthy discussions in Lesson 1 was to provide a solid conceptual foundation for understanding the mathematics of Lesson 2. If the question is confusing, you might want to review Lesson 1 or at least review the discussion pertaining to What is Heat? In Lesson 1, it was emphasized that heat is not something that is contained in an object. Objects do not contain heat. Objects, which are made of atoms, molecules and ions, contain energy. Heat is the transfer of energy from an object to its surroundings or to an object from its surroundings. So the question being asked on this page is what does this heat do to the object and to the surroundings when it is transferred? Like many questions in physics, it is a simple answer with deep meaning. Simple answers with deep meaning always exercise the brain. So put on your thinking cap and let's get to the answer.
Heat Changes the Temperature of Objects
What does heat do? First, it changes the temperature of an object. If heat is transferred from an object to the surroundings, then the object can cool down and the surroundings can warm up. When heat is transferred to an object by its surroundings, then the object can warm up and the surroundings can cool down. Heat, once absorbed as energy, contributes to the overall internal energy of the object. One form of this internal energy is kinetic energy; the particles begin to move faster, resulting in a greater kinetic energy. This  more vigorous motion of particles is reflected by a temperature increase. The reverse logic applies as well. Energy, once released as heat, results in a decrease in the overall internal energy of the object. Since kinetic energy is one of the forms of internal energy, the release of heat from an object causes a decrease in the average kinetic energy of its particles. This means that the particles move more sluggishly and the temperature of the object decreases. The release or absorption of energy in the form heat by an object is often associated with a temperature change of that object. This was the focus of the Thermometers as Speedometers in Lesson 1. What can be said of the object can also be said of the surroundings. The release or absorption of energy in the form heat by the surroundings is often associated with a temperature change of the surroundings. We often find that the transfer of heat causes a temperature change in both system and surroundings. One warms up and the other cools down.
more vigorous motion of particles is reflected by a temperature increase. The reverse logic applies as well. Energy, once released as heat, results in a decrease in the overall internal energy of the object. Since kinetic energy is one of the forms of internal energy, the release of heat from an object causes a decrease in the average kinetic energy of its particles. This means that the particles move more sluggishly and the temperature of the object decreases. The release or absorption of energy in the form heat by an object is often associated with a temperature change of that object. This was the focus of the Thermometers as Speedometers in Lesson 1. What can be said of the object can also be said of the surroundings. The release or absorption of energy in the form heat by the surroundings is often associated with a temperature change of the surroundings. We often find that the transfer of heat causes a temperature change in both system and surroundings. One warms up and the other cools down.
Heat Changes the State of Matter
But does the absorption or release of energy in the form of heat always cause a temperature change? Surprisingly, the answer is no. To illustrate why, consider the following situation, which is often demonstrated or even experimented with in a thermal physics unit in school. Para-dichlorobenzene, the main ingredient in many forms of mothballs, has a melting point of about 54 °C. Suppose that a sample of the chemical is collected in a test tube and heated to about 80°C. The para-dichlorobenzene will be in the liquid state (though much of it will have sublimed and be filling the room with a most noticeable aroma). Now suppose that a thermometer is inserted in the test tube and that the test tube is placed in a beaker of room temperature water. Temperature-time data can be collected every 10 seconds. Quite expectedly, one notices that the temperature of the para-dichlorobenzene gradually decreases. As heat is transferred from the high temperature test tube to the low temperature water, the temperature of the liquid para-dichlorbenzene decreases. But then quite unexpectedly, one would notice that this steady decrease in temperature ceases at about 54°C. Once the temperature of liquid para-dichlorbenzene decreases to 54°C, the thermometer level suddenly stands still. Based on the thermometer reading, you might think that no heat was being transferred. But a look in the test tube reveals dramatic change taking place. The liquid para-dichlorbenzene is crystallizing to form solid para-dichlorbenzene. Once the last trace of liquid para-dichlorbenzene vanishes (and it is in all solid form), the temperature begins to decrease again from 54°C to the temperature of the water. How can these observations help us to understand the question of what does heat do?
First, the decrease in temperature from 80°C to 54°C is easy to explain. We have learned in Lesson 1 that heat is transferred between two adjacent objects that are at different temperatures. The test tube and the para-dichlorbenzene are at a higher temperature than the surrounding water of the beaker. Heat will flow from the test tube of para-dichlorbenzene to the water, causing the para-dichlorbenzene to cool down and the water to warm up. And the decrease in temperature from 54°C to the temperature of the water in the beaker is also easily explainable. Two adjacent objects of different temperatures will transfer heat between them until thermal equilibrium is reached. The difficult explanation involves explaining what happens at 54°C. Why does the temperature no longer decrease when the liquid para-dichlorbenzene begins to crystallize? Is there still a transfer of heat between the test tube of para-dichlorbenzene and the beaker of water even when the temperature isn't changing?
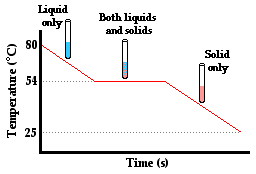 The answer to the question Is heat being transferred? is a resounding yes! After all, the principle is that heat is always transferred between two adjacent objects that are at different temperatures. A thermometer placed in the water reveals that the water is still warming up even though there is no temperature change in the para-dichlorbenzene. So heat is definitely being transferred from the para-dichlorbenzene to the water. But why does the temperature of the para-dichlorbenzene remain constant during this crystallization period? Before the para-dichlorbenzene can continue to lower its temperature, it must first transition from the liquid state to the solid state. The crystallization of para-dichlorbenzene occurs at 54°C - the freezing point of the substance. At this temperature, the energy that is lost by the para-dichlorbenzene is associated with a change in the other form of internal energy - potential energy. A substance not only possesses kinetic energy due to the motion of its particles, it also possesses potential energy due to the intermolecular attractions between particles. As the para-dichlorbenzene crystallizes at 54°C, the energy being lost is reflected by decreases in the potential energy of the para-dichlorbenzene as it changes state. Once all the para-dichlorbenzene has changed to the solid state, the loss of energy is once more reflected by a decrease in the kinetic energy of the substance; its temperature decreases.
The answer to the question Is heat being transferred? is a resounding yes! After all, the principle is that heat is always transferred between two adjacent objects that are at different temperatures. A thermometer placed in the water reveals that the water is still warming up even though there is no temperature change in the para-dichlorbenzene. So heat is definitely being transferred from the para-dichlorbenzene to the water. But why does the temperature of the para-dichlorbenzene remain constant during this crystallization period? Before the para-dichlorbenzene can continue to lower its temperature, it must first transition from the liquid state to the solid state. The crystallization of para-dichlorbenzene occurs at 54°C - the freezing point of the substance. At this temperature, the energy that is lost by the para-dichlorbenzene is associated with a change in the other form of internal energy - potential energy. A substance not only possesses kinetic energy due to the motion of its particles, it also possesses potential energy due to the intermolecular attractions between particles. As the para-dichlorbenzene crystallizes at 54°C, the energy being lost is reflected by decreases in the potential energy of the para-dichlorbenzene as it changes state. Once all the para-dichlorbenzene has changed to the solid state, the loss of energy is once more reflected by a decrease in the kinetic energy of the substance; its temperature decreases.
Heating Curves
So the second answer to the question What does heat do? is that it contributes to changes in state of a substance. Most students are familiar with at least three states of matter - solid, liquid and gas. The addition of heat to a sample of matter can cause solids to turn to liquids and liquids to turn to gases. Similarly, the removal of heat from a sample of matter can cause gases to turn to liquids and liquids to turn to solids. Each of these transitions between states occur at specific temperatures - commonly referred to as melting point temperature, freezing point temperature, boiling point temperature and condensation point temperature.
To further illustrate this relationship between heat transfer, temperature change and change of state, consider the following thought experiment. Suppose that a sample of water was placed in a Styrofoam cup with a digital thermometer. And suppose that the water is placed in the freezer (temperature = -20°C) and frozen. Suppose that the thermometer can be connected to a computer with software that is capable of collecting temperature-time data. After the water has frozen and remained in the freezer for several hours, it is removed and placed in a beaker on a hot plate. The hot plate is turned on, gets hot, and begins transferring energy in the form of heat to the beaker and the water. What changes would be observed in the temperature and the state of matter of the water over the course of time?
The diagram below depicts the so-called heating curve for the water. The heating curve represents the changes in temperature with respect to time for a sample of matter (such as the water) to which heat is transferred.
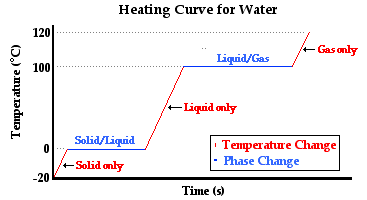
Observe that there are three sloped sections and two horizontal sections on the temperature-time plot. The first sloped section corresponds to a change in temperature of the ice from -20°C to 0°C. The water in its solid state is warming up to the melting point - the temperature at which water transitions between the solid and the liquid state. The heat transferred to the ice causes a temperature change. Once the transition temperature (melting point) of 0°C is reached, the heat added to the water causes the water to change from its solid state to its liquid state. This is referred to as melting. The melting occurs at a constant temperature. During this stage of the experiment, the energy absorbed by the water is used to loosen the attractions that hold one ice particle to another. Once all these attractions are loosened, the ice would be observed to have entirely melted. The contents of the Styrofoam cup are completely liquid. The next section of the heating curve is a sloped section. The liquid  water is increasing its temperature from 0°C to 100°C. The boiling point of water is 100°C; this is the temperature at which water transitions from the liquid state to the gaseous state. Once the sample of water reaches this temperature, boiling occurs. Large bubbles of gas would be observed forming throughout the bulk of the liquid. The heat added to the liquid during this stage of the thought experiment causes a loosening of the attractions that hold the water particles in the liquid state. The temperature remains constant while the state of water changes. Once all the water transitions from the liquid to the gaseous state, the sample of water (now in the gaseous state) begins to increase its temperature again.
water is increasing its temperature from 0°C to 100°C. The boiling point of water is 100°C; this is the temperature at which water transitions from the liquid state to the gaseous state. Once the sample of water reaches this temperature, boiling occurs. Large bubbles of gas would be observed forming throughout the bulk of the liquid. The heat added to the liquid during this stage of the thought experiment causes a loosening of the attractions that hold the water particles in the liquid state. The temperature remains constant while the state of water changes. Once all the water transitions from the liquid to the gaseous state, the sample of water (now in the gaseous state) begins to increase its temperature again.
In summary, the three sloped sections represent heat causing a temperature change in the substance that absorbs it. And the two plateau sections represent heat causing a change of state in the substance that absorbs it. An inquisitive student might ask, "What is the particle-level explanation of these changes?" (Thanks for asking.) The temperature changes are the result of the added energy causing the particles of water to move more vigorously. Either the particles of solid vibrate more vigorously about their fixed positions or the particles of liquid and gas move about their container more rapidly. Either way, the addition of heat is causing an increase in the average kinetic energy of the particles in the sample of water. The changes of state are the result of the added energy causing changes in the strength of the inter-particle attractions. The attractions that hold water in the solid or in the liquid state are being overcome. The energy is being used to loosen these attractions and change to a state of greater potential energy.
Flickr Physics Photo

(a) Water in a flask is heated to is boiling point. The gas exiting the flask cools while passing through the copper tubing. Condensed water droplets are seen exiting the end of the copper tube.
(b) The temperature of this condensed water is much less than 100°C. It is not hot enough to cause a burn.
(c) A bunsen burner flame is used to heat the condenser coils of the copper tube. This raises the temperature of the exiting water above the boiling point. It's gaseous water above 100°C that is exiting the copper tubing.
(d) This water vapor is so hot that it instantly ignites a match that is placed at its opening.
(e) Still being heated by the bunsen burner flame, the exiting water vapor is hot enough to scorch a sheet of paper ...
(f) ... and that spells phun for the people doing and watching the demonstration!
 Heat Does Work
Heat Does Work
So the transfer of energy in the form of heat is associated with changes in the temperature or changes in the state of a sample of matter. But is that all? Can heat do anything else? Once more, the answer is Yes! Energy transfer in the form of heat can result in the performance of work upon the system or the surroundings. Devices that utilize heat to do work are often referred to as heat engines. In general, an engine is a device that does work. A heat engine is a device that uses heat transfer as the source of energy for doing work.
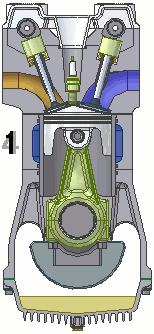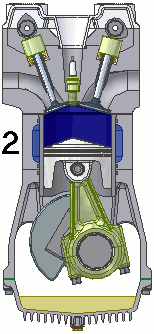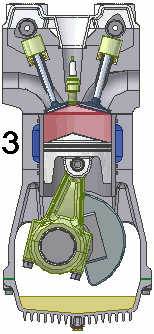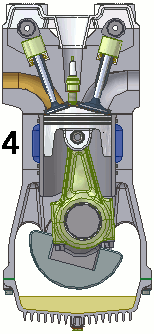
The internal combustion engine of an automobile is an example of a heat engine. Most internal combustion engines use a four-cycle process that is depicted in the animation at the right. As the fuel is burned (reacted with oxygen) in the engine, energy is released from the system of chemicals. There is a heat transfer from the hot system to the surrounding air of the cylinder. This transfer of heat to the air in the cylinder does work upon the piston, driving it downward. The piston is connected to the crankshaft of the car. The back and forth movement of the piston within the cylinder results in the rotational motion of the crankshaft and the generation of the energy required to set the car in motion. The internal combustion engine is an example of a heat engine. In this case, the internal energy stored in the chemical (gasoline) is converted to thermal energy (the flow of heat) that results in the performance of work. Heat engines will be discussed in greater detail in the Thermodynamics chapter of The Physics Classroom Tutorial. (Special thanks to UtzOnBike and the WikiMedia Commons for the animation of the four-cycle Otto engine as used above.)
Heat is the flow of energy from a high temperature location to a low temperature location. This flow of energy is always associated with changes in the system and the surroundings. There can be changes in the temperature, changes in the state of matter and changes that result from the doing of work. In the next section, we will look at the science of calorimetry. We will find that there is a very predictable set of mathematics associated with these changes. In fact, they are so predictable that scientists can use them to measure the amount of energy flow.