Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 3: Acids
Part b: Naming Acids Containing Oxyanions
Part 3a: Naming Acids (no O Atoms)
Part 3b: Naming Acids Containing Oxyanions
Oxyanions
 The previous page of Lesson 3 discussed the rules for naming and formula writing for acids that do not contain an oxygen atom. Now we will turn our attention to the names and formulae for acids containing oxygen. Like all acids, the acids of focus on this page have the generic formula of HA where A represents the anion that combines with hydrogen (H). The anion is known as an oxyanion, indicating that it is a negatively charged ion that contains at least one oxygen atom.
The previous page of Lesson 3 discussed the rules for naming and formula writing for acids that do not contain an oxygen atom. Now we will turn our attention to the names and formulae for acids containing oxygen. Like all acids, the acids of focus on this page have the generic formula of HA where A represents the anion that combines with hydrogen (H). The anion is known as an oxyanion, indicating that it is a negatively charged ion that contains at least one oxygen atom.
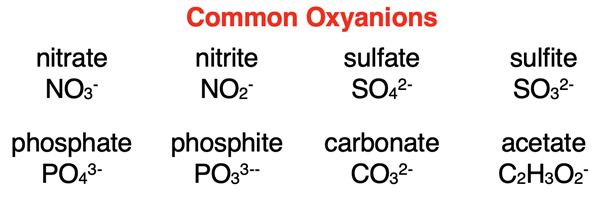
An inspection of our Polyatomic Ion List would reveal that most of the polyatomic ions contain oxygen. Here are the formulae and names of common ones. Observe that the names all have an -ate or -ite ending.
Nomenclature of Oxyacids
The rules for naming an acid are dependent upon whether the acid contains an oxyanion. Acids containing an oxyanion are referred to as oxyacids. The graphic below displays the names of several oxyacids.
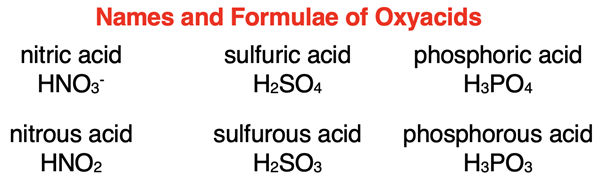
Some clear patterns emerge. There are two words. The second word is acid. The first word is the root name of the anion with either an -ic ending or an -ous ending. If the name of the anion ends -ate, then it is replaced with the -ic ending. And if the name of the anion ends -ite, then it is replaced with the -ous ending. The root name of an anion is typically the anion name with the ending removed. As discussed later, there are exceptions for sulfur and phosphorus containing oxyanions.

We can summarize this procedure as follows:
- Identify the formula of the anion.
- Use a Polyatomic Ion List to identify the full name of the anion.
- Identify the root name of the polyatomic ion.
- Add the -ic ending (for -ate ions) or the -ous ending (for -ite ions) to the root name.
- Add acid as the second word.
The table below shows several examples of how the name of an oxyacid is derived from the formula of the acid.
Rows c and d and rows e and f may include some surprises. It might be expected that the root name for sulfate and sulfite would be
sulf. And it might be expected that the root name for phosphate and phosphite would be
phosph. This is not the case. Instead,
sulfur and
phosphor are the root names for these two oxyanions. The
-ic and the
-ous are added to these root names as seen in rows c and d and rows e and f.
Names to Formulae
Identifying the formula that matches the name of an oxyacid involves working backwards. Begin by determining the anion formula (including the charge). Then determine the subscript that hydrogen will have. The subscript on hydrogen will be the charge of the oxyanion. Finally, write the formula for the oxyacid. Some examples are shown below.
Before You Leave
- Download our Study Card on Acids - Names and Formulae. Save it to a safe location and use it as a review tool. The Study Card covers all of Lesson 3, not just this page.
- The Check Your Understanding section below include questions and problems with answers and explanations and solutions. It provides a great chance to self-assess your understanding.
Check Your Understanding
Use the following questions to assess your understanding. Tap the
Check Answer buttons when ready.
1. Based on the formula, which of the following are oxyacids? Select all that apply.
- H2S
- HNO3
- NH3
- H3AsO4
- HC2H3O2
- NH4OH
2. Based on the formula, which of the following are oxyacids? Select all that apply.
- hydrosulfuric acid
- sulfuric acid
- phosphorous acid
- dihydrogen monoxide
- hydronitric acid
- potassium hydrogen phthalate
3. Write the name of the following oxyacids.
- HClO3
- HClO
- HMnO4
- H2Cr2O7
- HBrO3
- HBrO2