Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 2: Molecular Compounds
Part a: Properties of Molecular Compounds
Part 2a: Properties of Molecular Compounds
Part 2b: Names and Formulas of Molecular Compounds
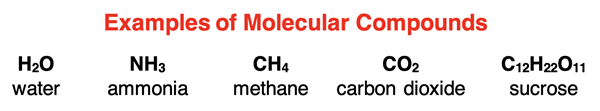
What is a Molecular Compound?
Lesson 1 of this chapter pertained to ionic compounds – compounds consisting of positive and negative ions. In Lesson 2, we will discuss a second class of compounds – molecular compounds. Molecular compounds are compounds consisting of two or more nonmetals. Unlike the ionic compounds of Lesson 1, molecular compounds do not consist of charged ions which attract each other. Instead, the atoms are bound together by a type of force known as a covalent bond to form a molecule. (Bonding will be discussed in full detail in Chapter 6.)
Examples of molecular compounds include H2O, CH4, NH3, CO2, PCl3, C12H11O22, etc. The elements in all these compounds are nonmetals. It might be surprising to read that hydrogen from Group 1 of the periodic table is described as a nonmetal. While hydrogen is located on the metal side of the periodic table, it in many ways acts more like a nonmetal than a metal. In Lesson 2, we will focus on binary molecular compounds – compounds consisting of only two nonmetal elements.
 At the particle level, molecular compounds are quite different than ionic compounds. The ions of an ionic compound are held together by strong electrical forces. The forces hold the ions in a highly organized structure known as a crystal lattice. The observable properties of ionic compounds are explained by these particle-level features. In contrast, molecular compounds do not consist of ions but rather of molecules. The molecules often do not assume a high degree of organization or structure. The forces that hold neighboring molecules together are considerably weaker than the forces that hold the ions of ionic compounds together. And at times, those forces in molecular compounds are so weak they can be considered negligible. The particle-level features of molecular compounds explain the observable physical properties that are discussed below.
At the particle level, molecular compounds are quite different than ionic compounds. The ions of an ionic compound are held together by strong electrical forces. The forces hold the ions in a highly organized structure known as a crystal lattice. The observable properties of ionic compounds are explained by these particle-level features. In contrast, molecular compounds do not consist of ions but rather of molecules. The molecules often do not assume a high degree of organization or structure. The forces that hold neighboring molecules together are considerably weaker than the forces that hold the ions of ionic compounds together. And at times, those forces in molecular compounds are so weak they can be considered negligible. The particle-level features of molecular compounds explain the observable physical properties that are discussed below.
Melting Point and Boiling Point
In simple terms, the melting point temperature is the temperature at which the solid state of a substance transitions into the liquid state. The boiling point is the temperature at which the state change between the liquid and gaseous state occurs. These two temperatures are related to the strength of inter-particle attractions for the substance. The transition from solid to liquid (melting) occurs when the particles vibrate with enough motion to overcome the forces that hold them in their solid state. Since the forces between molecules in a molecular compound are weaker than the forces between ions in an ionic compound, it takes less vibrational motion to free the molecules from their position. As such, the transition occurs at lower temperatures for molecular compounds than for ionic compounds. The same type of logic explains why molecular compounds have lower boiling points then ionic compounds. Since the inter-particle forces between molecules is weaker, the liquid particles require a lower degree of motion in order to turn into a gas.
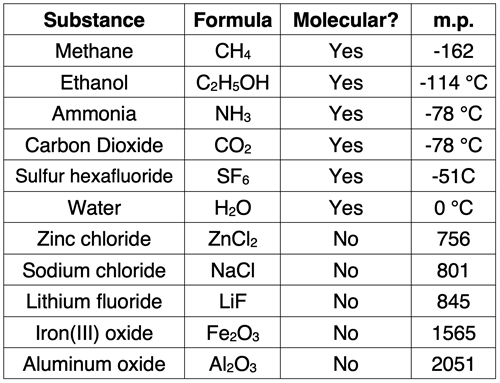
Ionic compounds exist as solids at typical room temperatures. On the other hand, molecular compounds can exist as a solid, liquid, or gas. But most commonly, binary molecular compounds are observed to exist as liquids or gases at room temperatures.
Soft and Flexible
We mentioned in Lesson 1 that ionic compounds are hard and brittle. These two properties were explained by the highly organized structure assumed by the ions and  the strong inter-particle forces that lock the ions in place. In contrast to ionic compounds, molecular compounds that are solid are soft and flexible. When an external force or stress is applied to a solid molecular substance, its tendency is to deform and not return to its original shape. The relatively weak inter-particle forces and lack of a rigid structure allow the molecules to reposition themselves in response to the external force. There are exceptions to this general rule. The exceptions usually arise when a solid molecular substance forms a more organized network or structure of molecules.
the strong inter-particle forces that lock the ions in place. In contrast to ionic compounds, molecular compounds that are solid are soft and flexible. When an external force or stress is applied to a solid molecular substance, its tendency is to deform and not return to its original shape. The relatively weak inter-particle forces and lack of a rigid structure allow the molecules to reposition themselves in response to the external force. There are exceptions to this general rule. The exceptions usually arise when a solid molecular substance forms a more organized network or structure of molecules.
Solubility
 Solubility refers to the amount of substance that dissolves in a solvent like water. Substances exhibit a wide range of water solubilities. In general, ionic compounds have high water solubilities. This indicates that a relatively large amount of the compound can dissolve in a fixed amount of water. The high solubility of ionic compounds in water is attributed to the fact that they consist of ions. The water molecules readily attract these ions and surround them, thus enhancing the tendency of the ionic compound to dissolve. On the other hand, molecular compounds have a generally lower solubility in water. This indicates that a relatively low amount of the compound is capable of dissolving in a fixed amount of water. We will revisit the topic of solubility and the dissolving process in the Solutions chapter of this Chemistry Tutorial.
Solubility refers to the amount of substance that dissolves in a solvent like water. Substances exhibit a wide range of water solubilities. In general, ionic compounds have high water solubilities. This indicates that a relatively large amount of the compound can dissolve in a fixed amount of water. The high solubility of ionic compounds in water is attributed to the fact that they consist of ions. The water molecules readily attract these ions and surround them, thus enhancing the tendency of the ionic compound to dissolve. On the other hand, molecular compounds have a generally lower solubility in water. This indicates that a relatively low amount of the compound is capable of dissolving in a fixed amount of water. We will revisit the topic of solubility and the dissolving process in the Solutions chapter of this Chemistry Tutorial.
Non-Electrolytes
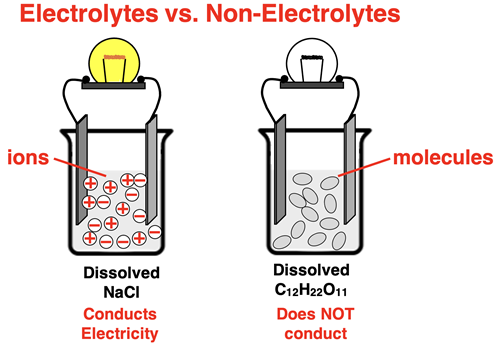
Ionic compounds like sodium chloride are considered electrolytes. When in the liquid state or dissolved in water, they conduct electricity. The property of being an electrolyte is explained by the fact that ionic compounds consist of electrically charged ions. These ions give the liquid or solution the ability to conduct a current. On the other hand, molecular compounds are non-electrolytes. In the liquid state or when dissolved in water, molecular compounds like sucrose do not conduct electricity. Molecular compounds are composed of neutral molecules. Being neutral, they do not contribute to the ability of the solution to conduct a current.
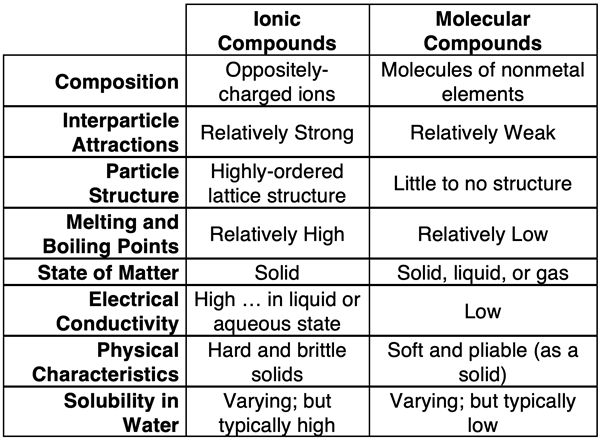
Comparison of Ionic and Molecular Compounds
The above discussion highlights the differences between ionic and molecular compounds. The differences are attributed to particle-level features of the two types of compounds. The table below provides a summary of the particle-level features and physical properties of the two types of compounds.
Check Your Understanding
Use the following questions to assess your understanding. Tap the Check Answer buttons when ready.
1. Classify each of the following compounds as being an ionic compound or a molecular compound.
- BH3
- AlF3
- Zn3P2
- P2O5
- SO2
- C2H6
2.
What am I?
Consider the following statements. Identify whether each statement best represents an ionic substance or a molecular substance.
- I boil at a temperature of 68 °C.
- I consist of carbon and oxygen atoms bound together.
- I form a hard, crystalline solid.
- Throw me in your fire pit and turn up the heat. I still won’t melt.
- I will conduct a current when dissolved in water.
- I can most commonly be found as a liquid or a gas at room temperature.
- Pour a teaspoon of me in a cup of water and stir. I will partly dissolve by you’ll see most of me at the bottom of the container in solid form.
- My particles are held together by very strong, electrical forces of attraction.