Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 3: Ions
Part b: Transition Metal Ions
Part 3a: Metals, Nonmetals, and Ions
Part 3b: Transition Metal Ions
Part 3c: Polyatomic Ions
Main Group vs. Transition Metal Elements
 The previous part of Lesson 3
The previous part of Lesson 3 focused on the formation of ions by main group elements of the periodic table. We learned that main group elements gain or lose electrons so as to acquire the stable electron configuration of a noble gas element. The metals lose electrons to become positive ions (cations) while the nonmetals gain electrons to become negative ions (anions). We also learned how to write the names and symbols of the ions and how to represent the change from a neutral atom to an ion using an ion formation equation.
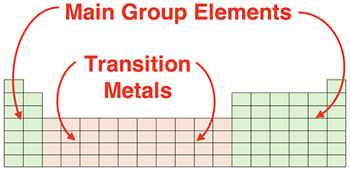
The number of electrons gained or lost by main group elements is highly predictable. The main group elements
ionize so as to become electronically identical to a noble gas. Such predictability is not the case for the transition metals. Being metals, the transition metals will lose electrons to become ions. And they thus become positive ions or cations. But the number of electrons that are lost is not always guided by the principle that the element wishes to become electronically identical to a noble gas. In fact, there simply is not a hard and fast rule for predicting the charge on a transition metal ion.
Transition Metal Ions
Transition metals are quite different than the metals of groups 1, 2, and 13. They can form more than one different ion, with each ion having a different charge. As an example, copper can lose one electron to form a 1+ ion or two electrons to from a 2+ ion.
Cu → Cu+ + e-
Cu → Cu2+ + 2 e-
Similarly, an atom of iron can form a 2+ ion or a 3+ ion by losing either two or three electrons.
Fe → Fe2+ + 2 e-
Fe → Fe3+ + 3 e-
These are just two of many examples. The fact is that most transition metal elements can form two or more different ions of varying charges.
 Names of Transition Metal Ions
Names of Transition Metal Ions
Because transition metals can form ions with different charges, there needs to be a means to distinguish between the various ions. The convention is to follow the element name by parenthesis. Roman numerals – I, II, III, IV, V, etc. – are included inside the parenthesis to indicate the amount of positive charge on the transition metal ion. Several examples are shown below.
As we continue to Chapter 4 of this
Chemistry Tutorial, we will learn the importance of the charges and names for transition metal ions. At that time, we will name compounds that are formed when such ions form so-called ionic compounds by combining with anions.
Check Your Understanding
Use the following questions to assess your understanding. Tap the
Check Answer buttons when ready.
1. Write the equation for the formation of the following transition metal ions from the corresponding atom.
- Scandium(III) ion
- Gold(III) ion
- Chromium(V) ion
New and Review
2. Reivew
isotope symbols. Then write the isotope symbol of the …
- zirconium(IV) ion that has 51 neutrons.
- manganese(V) ion that has 32 neutrons.
- silver(I) ion that has 61 neutrons.
3. Indicate the number of electrons in each of these isotopes:
- zirconium(IV) ion
- manganese(V) ion
- silver(I) ion