Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 4: Density
Part b: Measuring Density
Part 4a: Concepts and Mathematics of Density
Part 4b: Measuring Density
Determining the Density
 Density is a physical property of materials. It is relatively identifying since every material has a unique density value. But using a density measurement to identify an unknown substance is only possible if you have a reliable and accurate determination of the density.
Density is a physical property of materials. It is relatively identifying since every material has a unique density value. But using a density measurement to identify an unknown substance is only possible if you have a reliable and accurate determination of the density.
Density can be determined quite easily in a lab by determining the ratio of the mass to the volume. Mass measurements are straightforward using a digital balance. The volume of a liquid is easy to determine with a graduate cylinder or other calibrated instrument for determining volume. Volume measurements are a bit more complicated if the object is a solid or (worse) a solid with an irregular shape.
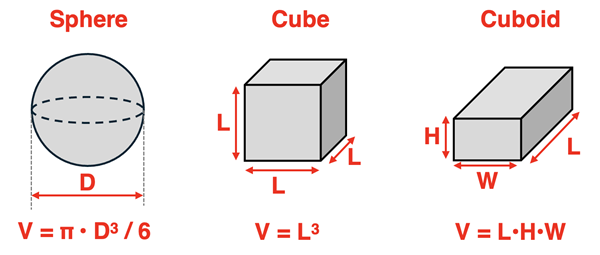
Measuring Volume using Geometry
Geometry can often be used to determine the volume of a solid object if the shape is regular and geometrically recognizable. For example, the volume of a solid object with the shape of a sphere, a cube, or a cuboid can be determined by making a one or more relevant measurements. For instance, measuring the diameter of a sphere or the edge lengths of a cube or cuboid and then using a volume formula for that specific shape will lead to a determination of the object’s volume.
Measuring Volume by Water Displacement
 The graduated cylinder is the go-to instrument for measuring liquid volumes in a high school chemistry lab. Suppose that you are doing a lab where you wish to determine the density of pre-1982 pennies. You add a stack of 10 pennies to 50.00 mL of water in a graduated cylinder. The pennies sink to the bottom of the cylinder and displace some of the water. The result is that the water level will rise upward. If the new water level is 53.50 mL, then the pennies have displaced 3.50 mL of water. The volume of the pennies is thus 3.50 mL. This is the method of water displacement. The table at the right show sample data for five stacks of 10 pennies each.
The graduated cylinder is the go-to instrument for measuring liquid volumes in a high school chemistry lab. Suppose that you are doing a lab where you wish to determine the density of pre-1982 pennies. You add a stack of 10 pennies to 50.00 mL of water in a graduated cylinder. The pennies sink to the bottom of the cylinder and displace some of the water. The result is that the water level will rise upward. If the new water level is 53.50 mL, then the pennies have displaced 3.50 mL of water. The volume of the pennies is thus 3.50 mL. This is the method of water displacement. The table at the right show sample data for five stacks of 10 pennies each.
 Measuring the volume of a solid object by water displacement is an exceptional method when the object has an irregular shape. The water displacement does not have to occur within a graduated cylinder. A beaker could first be filled with water to its overflow lip. Then the object is slowly submerged into the water. The water will be displaced and flow over the lip of the beaker. A second beaker can be used to collect the displaced water. The volume of this displaced water could then be measured with a graduated cylinder. Specialized displacement vessels can be purchased that make the collection of the displaced water considerably easier.
Measuring the volume of a solid object by water displacement is an exceptional method when the object has an irregular shape. The water displacement does not have to occur within a graduated cylinder. A beaker could first be filled with water to its overflow lip. Then the object is slowly submerged into the water. The water will be displaced and flow over the lip of the beaker. A second beaker can be used to collect the displaced water. The volume of this displaced water could then be measured with a graduated cylinder. Specialized displacement vessels can be purchased that make the collection of the displaced water considerably easier.
Before You Leave
- Need practice? Our Density Calculations 2 problem set at the Calculator Pad has several lab-based problems.
- The Check Your Understanding section below problems with answers and solutions. It is another great source of practice.
Check Your Understanding
1. In an effort to determine the density of an unknown metal, one of Mr. Glynch's lab groups massed several beads of the metal and determined its mass to be 65.54 grams. They placed 50.00 mL of water in a graduated cylinder. They added the beads to the water and observe that the water level rises to 57.79 mL. Determine the density of their unknown metal.
2. During the Dense Cents Lab, Jayzee and Lizz determine the mass of 30 pre-1982 pennies to be 93.08 g. They place the pennies in a graduated cylinder filled with 60.0 mL of water. The water level rises to 70.50 mL. According to their data, what is the density of a pre-1982 penny?
3. Anna Litical and Noah Formula are given a sample of pure metal that has a cubic shape. They measure the mass to be 34.28 g and the edge lengths to be 5.25 cm, 1.30 cm, and 1.85 cm. Use their date to determine the density of the unknown metal.