Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 3: Solids
Part c: Alloys
Part a:
Crystalline Solids
Part b:
Amorphous Solids
Part c: Alloys
What is  an Alloy?
an Alloy?
Lesson 3 of this chapter has been all about solids, their structure, bonding, and properties. The discussion would not be complete without a mention of alloys. Alloys are solid mixtures of two or more metallic elements. An alloy is not a compound with two metals chemically bonded to one another. Rather, an alloy is a homogeneous mixture of two or more metals. One of the metals is generally more abundant than the other metal; it is referred to as the base metal. The other element, known as the alloying element, is added to the base metal in an effort to modify the properties.
There are many familiar examples of alloys in the world that surrounds us. Steel is a mixture of iron and carbon atoms. Brass is a mixture of zinc and copper atoms. Sterling silver is a mixture of silver and copper. Bronze is a mixture of copper and tin. And solder is a mixture of lead and tin. These are a few of the many examples of alloys – solid mixtures of two or more metals.
Two Types of Alloys
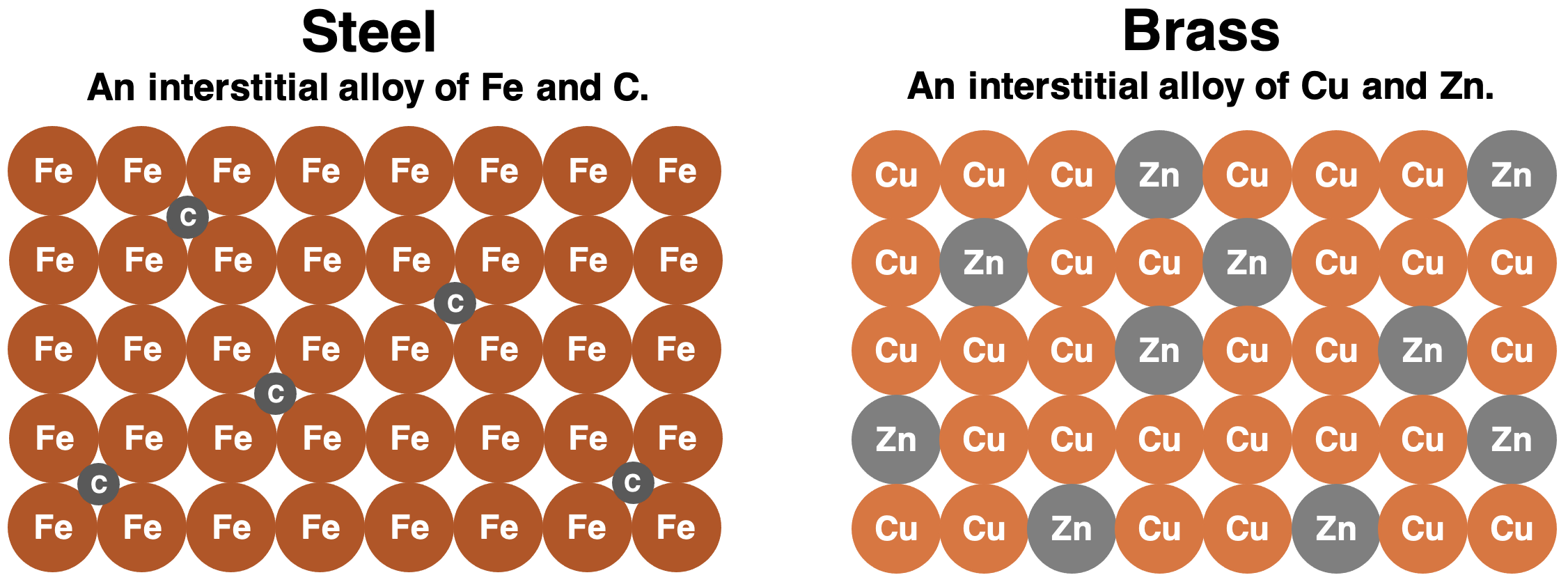
There are two types of metal alloys. One type is known as an interstitial alloy. This type is most common when the atoms of the two alloyed metals are considerably different sizes. The larger atoms occupy the lattice positions. In between these larger atoms are small gaps known as interstices. The smaller atoms occupy the space in these gaps. Steel is an example of an interstitial alloy. Iron is the base metal in steel. A small amount of carbon is mixed in with the iron. Iron is the larger atom (atomic number of 26), iron and occupies the lattice positions. Carbon is the smaller atom (atomic number of 6); it occupies some of the interstices between iron atoms.
The other type of metal alloy is known as a substitutional alloy. This type of alloy is most common when the atoms are similar in size. In a substitutional alloy, the alloyed metal replaces the atoms of the base metal in the crystal lattice. Similar to a substitution in an athletic event, atoms of the base metal are pulled out of the game and atoms of the alloyed metal are sent in to take their place. In order for the substitution to work without weakening the crystal lattice, the two alloyed metals must have approximately the same size. Brass is an example of a substitutional alloy. The base metal is copper; the copper is mixed with zinc. Depending on the desired properties, brass can consist of anywhere between 55% and 95% copper.
The most common manufacturing process for making alloys is known as the fusion method. The base metal is melted in a large furnace. The alloyed metal is then mixed in and melted with it. The two metals are thoroughly stirred to produce a homogenous mixture in the molten state. The mixture is then cast into a mold of the shape of the final product and allowed to cool and solidify. The rate at which the molten alloy is cooled can be controlled in order to affect the properties of the final product.

Source: Public Domain
Designer Metals
 By mixing two metal elements to from an alloy, chemists are able to produce products with properties that are different than either one of the pure metals. The types of properties that are desired include corrosion resistance, malleability (easily hammered, pressed, or rolled into a different shape), ductility (capable of being drawn into a wire), and strength. The percentages of the base metal and alloyed metal in the product affect the properties of the product and allow chemists to design products to meet specific marketplace needs.
By mixing two metal elements to from an alloy, chemists are able to produce products with properties that are different than either one of the pure metals. The types of properties that are desired include corrosion resistance, malleability (easily hammered, pressed, or rolled into a different shape), ductility (capable of being drawn into a wire), and strength. The percentages of the base metal and alloyed metal in the product affect the properties of the product and allow chemists to design products to meet specific marketplace needs.
Steel is mostly iron. By adding as much as 1.5% carbon to the base metal, the strength of the steel is maximized. This amount of steel is ideal for the making of tools. Lower carbon content can result in less strength but more malleability, allowing it to be more easily welded and shaped. Other metals can be added to the iron to increase strength (nickel) and hardness (manganese) or to provide corrosion resistance (chromium).
Check Your Understanding
Use the following questions to assess your understanding. Tap the Check Answer buttons when ready.
1. If boron (atomic number = 5) is added to iron (atomic number = 26) to produce an alloy, would it be an interstitial alloy or a substitutional alloy?
2. If manganese (atomic number = 25) is added to copper (atomic number = 29) to produce an alloy, would it be an interstitial alloy or a substitutional alloy?
3. If brass is thought of as a solution, then what is the solvent and what is the solute?