Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 3: Colligative Properties
Part a: Vapor Pressure Lowering
Part a: Vapor Pressure Lowering
Part b:
Boiling Point Elevation
Part c:
Freezing Point Depression
What is a Colligative Property?
 A colligative property is a property of a solution that depends on the number of particles of solute present and not upon the identity of the particles. We will discuss three types of colligative properties in Lesson 3. Those properties are referred to as vapor pressure lowering, freezing point depression, and boiling point elevation. A fourth property - osmotic pressure - will not be discussed in Lesson 3.
A colligative property is a property of a solution that depends on the number of particles of solute present and not upon the identity of the particles. We will discuss three types of colligative properties in Lesson 3. Those properties are referred to as vapor pressure lowering, freezing point depression, and boiling point elevation. A fourth property - osmotic pressure - will not be discussed in Lesson 3.
Suppose we have two aqueous solutions - one made by dissolving solute A and the other made by dissolving solute B. A property of the solution that does not depend on whether solute A or solute B is dissolved in the solvent … but does depend on how many particles of solute are present in the solution … is a colligative property. The solute particles that are dissolved in the solvent can be
- atoms (e.g., mercury atoms dissolved in water),
- ions (e.g., Na+ and Cl- ions dissolved in water), or
- molecules (e.g., sucrose molecules, C12H22O11, dissolved in water)
Colligative properties depend on the numbers of these particles and not upon whether the particles are Na
+ ions or C
12H
22O
11 molecules.
A Particle Model of Vapor Pressure
We discussed
a particle model of vapor pressure in
Chapter 11 of our
Chemistry Tutorial. We learned that there are two processes constantly occurring at the surface of any liquid – evaporation and condensation. These are opposite processes. Evaporation occurs when a particle of the liquid escapes the intermolecular attractions of other liquid particles and joins the gas state. If a liquid particle strikes the surface with enough kinetic energy, it can escape the pull of other liquid particles. Condensation involves particles of gas striking the liquid surface and experiencing intermolecular attractions to the liquid particles, pulling them into the liquid state. These opposing processes occur simultaneously and at equal rates (in closed systems).

Like any gas, the vapor above the liquid exerts a pressure upon the surface of the liquid. This pressure is referred to as
vapor pressure. Vapor pressure is greater when there are more vapor particles present in the space above the liquid. Evaporation rates increase with increasing temperature, explaining why
vapor pressure is greater at these temperatures. Any change that reduces the number of particles above the liquid surface will contribute to a decrease in vapor pressure. Adding a solute to the liquid is one means of reducing the vapor pressure of that liquid.
Effect of a Solute on Vapor Pressure
Let’s consider a sample of pure water at 25°C. Particles of water vapor above its surface exert a pressure of about 24 mm Hg on the water’s surface. We call this the vapor pressure of water.
Now suppose that dissolve sodium chloride (NaCl) in the water. It’s no longer pure water. Many of the water molecules are now surrounding the solute particles – the sodium and chloride ions. There is still a vapor pressure. But the vapor pressure is reduced by the presence of these dissolved ions. Why?
There are a couple of reasons why dissolved solute particles lower the vapor pressure. Both have to do with their ability to reduce the number of vapor particles above the liquid. They do so by reducing the rate of evaporation of the solvent. There are two ways that they do this. First, solute particles now occupy some space at the water’s surface, interfering with water molecules that could strike the surface and become a vapor. Second,
solute particles are interacting with the solvent particles. The dissolved ions and the water engage in
ion-dipole interactions. With some water molecules now pre-occupied with the solvation process, they are less involved in the evaporation process.
Vapor Pressure Lowering and the Number of Solute Particles
The two effects described above that reduce evaporation rates become more pronounced when the concentration of dissolved particles increases. A 1.00 M solution of NaCl
(aq) will lower the vapor pressure more than a 0.10 M solution lowers it. This is the nature of a colligative property – it depends upon the number of solute particles dissolved in the solution.
Solute concentration is not the only variable that must be considered. This is a particle phenomenon and it is the concentration of the dissolved particles that matters. Molecular solids like C
12H
22O
11 (sucrose) have a different effect than ionic solutes like NaCl. The dissolving of one particle of sucrose results in a single dissolved particle:
C12H22O11(s) → C12H22O11(aq)
But
ionic solutes dissociate when they dissolve. The dissolving of a single NaCl unit results in two dissolved particles – a sodium ion and a chloride ion.
NaCl(s) → Na+(aq) + Cl-(aq)
Each ion is solvated by water molecules, occupies space at the surface of the water, and reduces the vapor pressure of the solution by twice the amount of a single molecule of sugar.
Then there is aluminum chloride – AlCl
3. The dissolving of a single AlCl
3 unit results in four dissolved particles – one aluminum ion and three chloride ions.
AlCl3(s) → Al3+(aq) + 3 Cl-(aq)
An aqueous solution of aluminum chloride is four times as effective as a solution of sucrose at lowering the vapor pressure and twice as effective as a solution of sodium chloride. When we say a colligative property depends upon the number of solute particles present in the solution, we are referring to the atoms, ions, or molecules that are being solvated by the solvent. If
dissociation occurs, it is important to count the individual ions as the particles.
Raoult’s Law
There is a mathematical model that coordinates with the particle model presented above. The mathematical model is known as
Raoult’s Law. It describes the quantitative relationship between the amount of vapor pressure lowering and the number of dissolved particles in the solution. Like many mathematical models, it is based on some assumptions. It assumes that the solute is non-volatile and does not make any contribution to the overall vapor pressure of the solution. The model also assumes an ideal solution - a solution in which the solute-solvent interparticle attractions are comparable in strength to the solute-solute and solvent-solvent attractions.
Raoult’s Law predicts that the vapor pressure of a solution (
Psolution) is equal to the mole fraction of the solvent (
Χsolvent) in the solution multiplied by the vapor pressure of the pure solvent (
Ppure solvent).
Psolution = Χsolvent • Ppure solvent
There are at least two components in every solution - a solute and a solvent. The total number of particles (or moles of particles) is the sum of all the solute particles and solvent particles. A mole fraction is a concentration unit that indicates the fraction of the total number of particles that is a solute (
Χsolute) or a solvent (
Χsolvent). These two mole fractions add to 1.00.
Χsolute + Χsolvent = 1.00
A pure solvent would have a mole fraction of
Χsolvent = 1.00 and for a solution,
Χsolvent must always be less than 1.00.
Adding more and more solute to a solution increases the value of
Χsolute and reduces the value of
Χsolvent. In turn, adding more and more solvent causes the vapor pressure of the solution (
Psolution) to decrease more and more. The amount of
vapor pressure lowering is defined as
∆P.
∆P = Ppure solvent - Psolution
This equation can be manipulated to develop and equation for vapor pressure lowering (
∆P) expressed as a function of the mole fraction of the solute (
Χsolute). First, Raoult’s Law is used to substitute
Χsolvent • Ppure solvent for
Psolution.
∆P = Ppure solvent - Χsolvent • Ppure solvent
Then
Ppure solvent is factored out of each term on the right side.
∆P = ( 1 - Χsolvent ) • Ppure solvent
The quantity
1 - Χsolvent is equal to the mole fraction of the solute (
Χsolute). After making the substitution, we have an equation that relates the amount of vapor pressure lowering (
∆P) to the mole fraction of the solute (
Χsolute) and the vapor pressure of the pure solvent (
Ppure solvent).
∆P = Χsolute • Ppure solvent
Some Graphical Representations
There are a few common graphical representations of these Raoult’s Law relationships. One is a simple plot of the vapor pressure of a solution (
Psolution) as a function of the mole fraction of the solvent (
Χsolvent). As you would expect, the vapor pressure value is equal to the vapor pressure of the pure solvent when the mole fraction of the solvent is 1.00.
A second similar representation is to display the vapor pressure as a function of the mole fraction of the solute (
Χsolute). As shown below, the vapor pressure decreases as the mole fraction of the solute increases.
A third graphical representation is a plot of the actual amount of vapor pressure lowering (
∆P) as a function of the mole fraction of solute (
Χsolute). As you would expect, the amount of lowering increases with increasing mole fraction.
Vapor pressure lowering is the first of three colligative properties that we discuss in Lesson 3. On
the next page we will discuss a second colligative property - boiling point elevation.
Before You Leave
- Download our Study Card on Colligative Properties. Save it to a safe location and use it as a review tool. (Coming Soon.)
- The Check Your Understanding section below include questions with answers and explanations. It provides a great chance to self-assess your understanding.
Check Your Understanding
Use the following questions to assess your understanding. Tap the Check Answer buttons when ready.
1. Describe what makes a colligative property uniquely different than a property that is not colligative.
2. When it comes to a colligative property, how is the solute carbon dioxide dissolving in an aqueous solution different than a solute like calcium chloride dissolving in an aqueous solution?
3. Consider Diagrams A and B below. Diagram A shows a sample of pure water. Diagram B shows a sample of NaCl(aq).
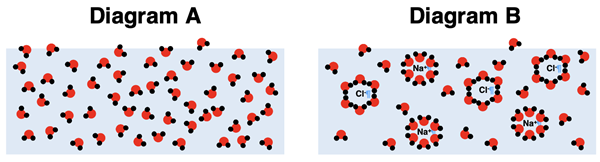
Which sample has the greatest vapor pressure? Use a particle model to explain why there is a difference in vapor pressure between the two samples.
4. Rank the following samples in order of increasing vapor pressure, from lowest to highest. (Assume no difference in temperature.)
- Sample A: Pure Water
- Sample B: 0.25 M NaF(aq)
- Sample C: 0.25 M C12H22O11(aq)
5. Rank the following samples in order of increasing vapor pressure, from lowest to highest. (Assume no difference in temperature.)
- Sample A: 0.25 M NaCl(aq)
- Sample B: 2.50 M NaCl(aq)
- Sample C: 0.75 M NaF(aq)
6. Rank the following samples in order of increasing vapor pressure, from lowest to highest. (Assume no difference in temperature.)
- Sample A: 1.00 M MgCl2(aq)
- Sample B: 1.00 M NaCl(aq)
- Sample C: 1.00 M FeCl3(aq)