Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
 As discussed in Unit 10 of The Physics Classroom Tutorial, electromagnetic waves are waves that are capable of traveling through a vacuum. Unlike mechanical waves that require a medium in order to transport their energy, electromagnetic waves are capable of transporting energy through the vacuum of outer space. Electromagnetic waves are produced by a vibrating electric charge and as such, they consist of both an electric and a magnetic component. The precise nature of such electromagnetic waves is not discussed in The Physics Classroom Tutorial. Nonetheless, there are a variety of statements that can be made about such waves.
As discussed in Unit 10 of The Physics Classroom Tutorial, electromagnetic waves are waves that are capable of traveling through a vacuum. Unlike mechanical waves that require a medium in order to transport their energy, electromagnetic waves are capable of transporting energy through the vacuum of outer space. Electromagnetic waves are produced by a vibrating electric charge and as such, they consist of both an electric and a magnetic component. The precise nature of such electromagnetic waves is not discussed in The Physics Classroom Tutorial. Nonetheless, there are a variety of statements that can be made about such waves.
Electromagnetic waves exist with an enormous range of frequencies. This continuous range of frequencies is known as the electromagnetic spectrum. The entire range of the spectrum is often broken into specific regions. The subdividing of the entire spectrum into smaller spectra is done mostly on the basis of how each region of electromagnetic waves interacts with matter. The diagram below depicts the electromagnetic spectrum and its various regions. The longer wavelength, lower frequency regions are located on the far left of the spectrum and the shorter wavelength, higher frequency regions are on the far right. Two very narrow regions within the spectrum are the visible light region and the X-ray region. You are undoubtedly familiar with some of the other regions of the electromagnetic spectrum.
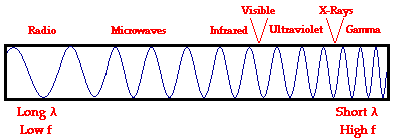
Visible Light Spectrum
The focus of Lesson 2 will be upon the visible light region - the very narrow band of wavelengths located to the right of the infrared region and to the left of the ultraviolet region. Though electromagnetic waves exist in a vast range of wavelengths, our eyes are sensitive to only a very narrow band. Since this narrow band of wavelengths is the means by which humans see, we refer to it as the visible light spectrum. Normally when we use the term "light," we are referring to a type of electromagnetic wave that stimulates the retina of our eyes. In this sense, we are referring to visible light, a small spectrum from the enormous range of frequencies of electromagnetic radiation. This visible light region consists of a spectrum of wavelengths that range from approximately 700 nanometers (abbreviated nm) to approximately 400 nm. Expressed in more familiar units, the range of wavelengths extends from 7 x 10-7 meter to 4 x 10-7 meter. This narrow band of visible light is affectionately known as ROYGBIV.
Each individual wavelength within the spectrum of visible light wavelengths is representative of a particular color. That is, when light of that particular wavelength strikes the retina of our eye, we perceive 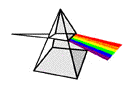 that specific color sensation. Isaac Newton showed that light shining through a prism will be separated into its different wavelengths and will thus show the various colors that visible light is comprised of. The separation of visible light into its different colors is known as dispersion. Each color is characteristic of a distinct wavelength; and different wavelengths of light waves will bend varying amounts upon passage through a prism. For these reasons, visible light is dispersed upon passage through a prism. Dispersion of visible light produces the colors red (R), orange (O), yellow (Y), green (G), blue (B), and violet (V). It is because of this that visible light is sometimes referred to as ROY G. BIV. (Incidentally, the indigo is not actually observed in the spectrum but is traditionally added to the list so that there is a vowel in Roy's last name.) The red wavelengths of light are the longer wavelengths and the violet wavelengths of light are the shorter wavelengths. Between red and violet, there is a continuous range or spectrum of wavelengths. The visible light spectrum is shown in the diagram below.
that specific color sensation. Isaac Newton showed that light shining through a prism will be separated into its different wavelengths and will thus show the various colors that visible light is comprised of. The separation of visible light into its different colors is known as dispersion. Each color is characteristic of a distinct wavelength; and different wavelengths of light waves will bend varying amounts upon passage through a prism. For these reasons, visible light is dispersed upon passage through a prism. Dispersion of visible light produces the colors red (R), orange (O), yellow (Y), green (G), blue (B), and violet (V). It is because of this that visible light is sometimes referred to as ROY G. BIV. (Incidentally, the indigo is not actually observed in the spectrum but is traditionally added to the list so that there is a vowel in Roy's last name.) The red wavelengths of light are the longer wavelengths and the violet wavelengths of light are the shorter wavelengths. Between red and violet, there is a continuous range or spectrum of wavelengths. The visible light spectrum is shown in the diagram below.
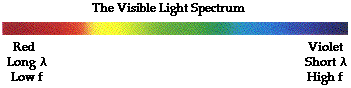
When all the wavelengths of the visible light spectrum strike your eye at the same time, white is perceived. The sensation of white is not the result of a single color of light. Rather, the sensation of white is the result of a mixture of two or more colors of light. Thus, visible light - the mix of ROYGBIV - is sometimes referred to as white light. Technically speaking, white is not a color at all - at least not in the sense that there is a light wave with a wavelength that is characteristic of white. Rather, white is the combination of all the colors of the visible light spectrum. If all the wavelengths of the visible light spectrum give the appearance of white, then none of the wavelengths would lead to the appearance of black. Once more, black is not actually a color. Technically speaking, black is merely the absence of the wavelengths of the visible light spectrum. So when you are in a room with no lights and everything around you appears black, it means that there are no wavelengths of visible light striking your eye as you sight at the surroundings.
Investigate!
The widget below matches the wavelength of light (in nanometers) to a particular color of light. Explore by entering various values between 400 nanometers and 700 nanometers. Values outside this range are not visible and therefore not associated with human-perceived color.
Check Your Understanding
1. A light wave is an electromagnetic wave that has both an electric and magnetic component associated with it. Electromagnetic waves are often distinguished from mechanical waves. The distinction is based on the fact that electromagnetic waves ______.
a. can travel through materials and mechanical waves cannot
b. come in a range of frequencies and mechanical waves exist with only certain frequencies
c. can travel through a region void of matter and mechanical waves cannot
d. electromagnetic waves cannot transport energy and mechanical waves can transport energy
e. electromagnetic waves have an infinite speed and mechanical waves have a finite speed
2. Consider the electromagnetic spectrum as you answer these three questions.
a. Which region of the electromagnetic spectrum has the highest frequency?
b. Which region of the electromagnetic spectrum has the longest wavelength?
c. Which region of the electromagnetic spectrum will travel with the fastest speed?
3. Consider the visible light spectrum as you answer these two questions.
a. Which color of the visible light spectrum has the greatest frequency?
b. Which color of the visible light spectrum has the greatest wavelength?