Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 2: Covalent Bonding
Part c: Double and Triple Bonds
Part 2a:
Covalent Bonds
Part 2b:
Lewis Electron Dot Structures
Part 2c: Double and Triple Bonds
Part 2d:
Octet Breakers
Part 2e:
Formal Charge Considerations
Multiple Bonds
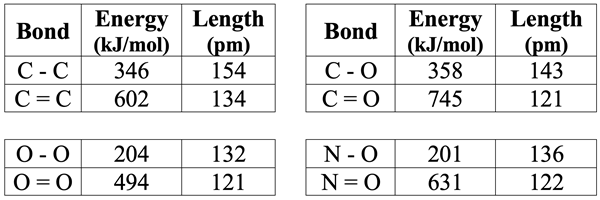
 So far in this chapter we have only seen single bonded atoms. Single bonds are the result of a single electron pair being shared between two atoms. They are often represented by a line between atoms on an electron dot diagram. There are also double bonds and triple bonds. A double bond is the result of two electron pairs being shared between two atoms. With four electrons being shared, the electrostatic attraction between atoms is stronger than the case of a single bond. The double-bonded atoms are drawn closer together, resulting in a shorter bond length and a greater bond energy. A triple bond involves three electron pairs being shared between two atoms. This results in an even greater attraction between atoms, an even shorter bond, and an even greater bond energy. Double bonds are often represented on dot diagrams by a pair of bond lines. Triple bonds are represented by three lines between bonded atoms. Estimated bond energies and bond lengths for a variety of single and double bonds are shown below.
So far in this chapter we have only seen single bonded atoms. Single bonds are the result of a single electron pair being shared between two atoms. They are often represented by a line between atoms on an electron dot diagram. There are also double bonds and triple bonds. A double bond is the result of two electron pairs being shared between two atoms. With four electrons being shared, the electrostatic attraction between atoms is stronger than the case of a single bond. The double-bonded atoms are drawn closer together, resulting in a shorter bond length and a greater bond energy. A triple bond involves three electron pairs being shared between two atoms. This results in an even greater attraction between atoms, an even shorter bond, and an even greater bond energy. Double bonds are often represented on dot diagrams by a pair of bond lines. Triple bonds are represented by three lines between bonded atoms. Estimated bond energies and bond lengths for a variety of single and double bonds are shown below.
On this part of Lesson 2 we will learn when and how to include double and triple bonds on Lewis electron dot diagrams.
Drawing Electron Dot Diagrams Revisited
 Previously in Lesson 2 we learned a method for drawing Lewis electron dot diagrams. The method involved determining the number of valence shell electrons in a molecule, drawing its skeleton structure, and then populating all atoms with an octet of electrons. The fourth and most important step involved counting the number of electrons used in the diagram and comparing it to the number of valence shell electrons. If the number of electrons used is greater than the number of valence electrons, then adjustments must be made. The adjustment involves removing two electrons (or more) and then creating a double bond or a triple bond. In our method, this is step 4b. (If not familiar with our method, you may wish to review it and the examples on the previous page.) The examples on this page demonstrate this method being practiced. The examples progress from the simple to the complex.
Previously in Lesson 2 we learned a method for drawing Lewis electron dot diagrams. The method involved determining the number of valence shell electrons in a molecule, drawing its skeleton structure, and then populating all atoms with an octet of electrons. The fourth and most important step involved counting the number of electrons used in the diagram and comparing it to the number of valence shell electrons. If the number of electrons used is greater than the number of valence electrons, then adjustments must be made. The adjustment involves removing two electrons (or more) and then creating a double bond or a triple bond. In our method, this is step 4b. (If not familiar with our method, you may wish to review it and the examples on the previous page.) The examples on this page demonstrate this method being practiced. The examples progress from the simple to the complex.
Example: O2 Molecule
Steps 1-3 and the start of Step 4 of our method are shown below:

It does not matter which electron pair is removed. But since there are two too many electrons in the dot diagram, an electron pair will have to be discarded. This is shown below.

Removing the electron pair fixes a problem but creates a new problem. There are now the correct number of electrons in the diagram. But the oxygen on the right is two electrons short of an octet. We need to get it two more electrons without adding any more electrons to the diagram. The only way to do that is to move a lone pair off the left O atom to a location in between the atoms such that it is a shared pair. Doing this satisfies the right O atom’s need for 8 electrons without removing an electron pair from the left O atom. This is shown below.

Now check the final diagram with two questions in mind:
- Does every atom satisfy the octet rule?
- Is the number of electrons in the diagram equal to the number of valence electrons?
If both answers are yes (and they are), then you have the correct Lewis electron dot diagram.
Drawing Lewis diagrams takes practice. And lots of it. And it takes a commitment to a logical method. We have provided the method. (There are other methods that work as well.) And we will provide plenty of worked out examples for you to see the method being implemented. The next two examples will be similar with some twists.
Example: N2 Molecule
Steps 1-3 and the start of Step 4 of our method are shown below:
It does not matter which two electron pairs are removed. But since there are four too many electrons in the dot diagram, two pairs must be discarded. This is shown below.
Once more removing the two electron pairs fixes a problem – we now have the correct number of electrons in the diagram. But it creates a new problem of the nitrogen on the right being four electrons short of an octet. We need to get this nitrogen four more electrons without adding any more electrons to the diagram. The only way to do that is to move two lone pairs off the left N atom to a location in between the atoms such that they become shared pairs. A triple bond is formed. Doing this satisfies the right N atom’s need for 8 electrons without removing any electron pairs from the left N atom. This is shown below.
Now check the final diagram with two questions in mind:
- Does every atom satisfy the octet rule?
- Is the number of electrons in the diagram equal to the number of valence electrons?
We can answer yes to both questions. The Lewis diagram is now complete.
Example: CO2 Molecule
Steps 1-3 and the start of Step 4 of our method are shown below:
It does not matter which two electron pairs are removed. But since there are four too many electrons in the dot diagram, two pairs must be discarded. We have chosen to remove the two pairs on the central carbon. This is shown below.
The central C atom now needs to have four more electrons but they can’t be added from
nowhere. The central C atom needs to get four electrons from the existing lone pairs on the O atoms. There’s more than one way that this can be done. But the best way is to move a lone pair from the left oxygen to a location between the left O atom and the C atom to form a double bond. Then repeat the same for the right O atom, moving a long pair to a location between the right O and the central C to form a second double bond. This is shown below.
Now it was mentioned that there was more than one way to satisfy the octet of the central carbon but that the above method was the best way. So, what’s the other way and why is it not best or preferred? The second method involves moving two electron pairs from the same oxygen (either one) to a location between that oxygen and the carbon atom to form a triple bond. This is shown below.
Doing this satisfies the octet of every atom and uses the same number of electrons as the number of valence shell electrons determined in step 1. So why doesn’t this happen? The full answer can get complicated and will have to wait until
Lesson 2e when we discuss formal charge considerations. But a quick way of addressing this is to recall that O atoms generally form two bonds and have two lone pairs. There’s no law that states that O atoms must have four electrons involved in bonding and four electrons as lone pairs. And there’s plenty of instances in which we may see O atoms not following this
non-law. But you can count on the most stable configuration of bonds for oxygen being in situations in which it has two lone pairs. A full discussion of this will come in
Lesson 2e. For now, rule out the diagram below.
Example: SO2 Molecule
Steps 1-3 and the start of Step 4 of our method are shown below:
It does not matter which electron pair is removed. But since the number of electrons in the diagram exceeds the number of valence electrons by two, one pair must be removed. We have chosen to remove a pair from the central sulfur atom. This is shown below.
The central S atom now needs to have two more electrons. The electrons must be moved from an existing unshared pair on one of the oxygen atoms. We have chosen to move an electron pair from the left O atom to a location between it and the S atom to form a double bond. This ensures that the S atom has an octet and that the left O atom still has an octet. This is shown below.
Those final two questions are always important:
- Does every atom satisfy the octet rule?
- Is the number of electrons in the diagram equal to the number of valence electrons?
Since can answer yes to both questions. The Lewis diagram is now complete.
Resonance Structures
Questions often arise when students see all the electron maneuvering shown above. Does it matter that you moved the electron from the left O atom and not the right O atom? Wouldn’t the result be different if a different electron pair had been removed? We have three replies:
- What really ultimately matters is: a) Every atom satisfies the octet rule. b) And the number of electrons in the diagram equals the number of valence shell electrons.
- Drawing electron dot diagrams is not a very good spectator sport. You need to practice lots and lots of them. Practice the same molecule multiple times and try it different ways. Soon you realize the patterns by which the electron removal and maneuvering is done. Your confidence grows as your experience the realization of these patterns.
- There’s something known as a resonance structure. Let’s talk about it.
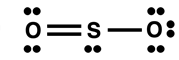
An inspection of the Lewis electron dot diagram at the right would infer that the molecule has two different S-O bonds. One is a single bond and the other is a double bond. As discussed at the top of the page, these two bonds would have different bond energies and different bond lengths. But surprisingly when the molecule SO
2 is analyzed in a lab, we find that there is only one type of bond. And we find its bond length is in between the expected bond length for an S-O single bond and an S-O double bond. Chemists describe the structure as a
resonance between two different structures. They represent the idea by drawing both structures with a double-headed arrow between them.
The actual structure is neither the one on the left nor the one on the right. The actual structure is an
average or a
hybrid of these two structures.
The issue with SO
2 is related to the fact that the Lewis electron dot model assumes that electrons are localized on atoms or between two atoms. Chemists believe that resonance situations arise because there are situations in which electrons are actually delocalized. That is, sometimes electrons can be spread across three or more atoms and not confined to a single atom or the space between two bonded atoms. The concept of
resonance structures are required by our localized electron model to describe these delocalized electrons. We will have more to say about this topic in
Lesson 3 when we introduce the Hybridized Atomic Orbital Model.
Example: NO3- Ion
We briefly discussed
electron dot diagrams for polyatomic ions on the previous page. They are done using the same method but the ion charge has to be accounted for when determining the number of valence electrons. Steps 1-3 and the start of Step 4 for our first ion are shown below:
In Step 3, there is a convention that arises for the first time in this chapter. When a Lewis electron dot diagram is drawn for an ion, the diagram is enclosed in brackets and the ion charge is listed in a superscripted location outside the brackets. This is a means of communicating that the dot diagram describes the NO
3- ion and not the NO
3 molecule.
Now let’s fix our dot diagram by first removing an electron pair. You can remove any electron pair you wish. We’ve removed the electron pair on the central N atom. This is shown below.
The central N atom now needs to have two more electrons. The electrons must be moved from an existing unshared pair on one of the oxygen atoms. We have chosen to move an electron pair from the left O atom to a location between it and the N atom to form a double bond. This ensures that both the N atom and the left O atom have an octet. This is shown below.
Once you have done the maneuvering of electrons, evaluate your electron dot diagram with two questions.
- Does every atom satisfy the octet rule?
- Is the number of electrons in the diagram equal to the number of valence electrons?
We can answer yes to both questions. The Lewis diagram is now complete.
One final thing to note about the NO
3- ion. The molecule exhibits resonance. Studies of the actual molecule suggest that there is only one type of N-O bond. The actual structure must be a hybrid resonance of the following three dot diagrams.
Example: CO32- Ion
Our final example is for another ion. Steps 1-3 and the start of Step 4 for the carbonate ion are shown below:
We will remove an electron pair from the central carbon atom in order to match the number of electrons in the diagram to the number of valence electrons. We’ve removed the electron pair on the central C atom. This is shown below.
The central C atom now needs to have two more electrons. The electrons must be moved from an existing unshared pair on one of the neighboring oxygen atoms. We have chosen to move an electron pair from the left O atom to a location between it and the C atom to form a double bond. This ensures that both the C atom and the left O atom have an octet. This is shown below.
Once you have done the maneuvering of electrons, evaluate your electron dot diagram with two questions.
- Does every atom satisfy the octet rule?
- Is the number of electrons in the diagram equal to the number of valence electrons?
We can answer yes to both questions. The Lewis diagram is now complete.
Finally note that this is a resonance situation again in which the actual arrangement of electrons is a hybrid of three possible diagrams.
If you’ve been following along with Lesson 2, then your know-how and skill with drawing Lewis diagrams should be improving. We have one more issue to address: what happens when you’ve finished step 3 and the number of electrons in the diagram is less than the number of valence shell electrons? We will address this question in
Lesson 2d.
Before You Leave
Check Your Understanding
Use the following questions to assess your understanding. Tap the Check Answer buttons when ready.
1. Construct a Lewis electron dot diagram for the O3 molecule.
2. Construct a Lewis electron dot diagram for the SO
3 molecule.
3. Construct a Lewis electron dot diagram for the NO
- ion.
4. Construct a Lewis electron dot diagram for the CN
- ion.
5. Construct a Lewis electron dot diagram for the NO
2- ion.
6. For which of the above situations do resonance structures exist?