Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 2: Covalent Bonding
Part d: Octet Breakers
Part 2a:
Covalent Bonds
Part 2b:
Lewis Electron Dot Structures
Part 2c:
Double and Triple Bonds
Part 2d: Octet Breakers
Part 2e:
Formal Charge Considerations
Why is There an Octet Rule?
The octet rule describes the general tendency of atoms of main group elements to form bonds in order to acquire eight valence shell electrons. But why exactly eight? What is so important about 8 electrons?
Noble gas elements in group 18 are observed to be the most electronically stable elements on the periodic table. It is well understood that atoms form bonds to increase their stability. Noble gas elements seldom form bonds. We thus conclude that they are already electronically stable.
The electron configuration of every noble gas element ends with ns2np6 where n is the period number. As examples, neon is [He]2s22p6 and argon is [Ne]3s23p6. (Helium is the exception since there are no p orbitals in the n=1 energy level.) This configuration corresponds to a set of s and p orbitals being completely filled with electrons. Since an s orbital holds two electrons and the three p orbitals can hold a total of six electrons, the total capacity of the s and p orbitals is eight. These orbitals are observed to most commonly be involved in bonding. And the electrons in these orbitals are referred to as valence shell electrons. The octet rule is based on this understanding.
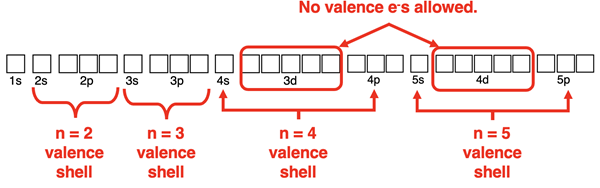
Expanded Octets
 Elements in the third row of the periodic table and beyond have d orbitals available for bonding. These orbitals are higher in energy than the s and the p orbitals of the corresponding energy level. That is, 3d orbitals are higher in energy than 3s and 3p orbitals. And 4d orbitals are higher in energy than 4s and 4p orbitals. Argon, the noble gas element in the third row does not use the 3d orbitals to hold electrons. And krypton, the noble gas element in the fourth row does not use the 4d orbitals to hold electrons.
Elements in the third row of the periodic table and beyond have d orbitals available for bonding. These orbitals are higher in energy than the s and the p orbitals of the corresponding energy level. That is, 3d orbitals are higher in energy than 3s and 3p orbitals. And 4d orbitals are higher in energy than 4s and 4p orbitals. Argon, the noble gas element in the third row does not use the 3d orbitals to hold electrons. And krypton, the noble gas element in the fourth row does not use the 4d orbitals to hold electrons.
There are situations in which a central atom in a molecule needs to exceed its octet. For instance, the P atom of PCl5 forms five covalent bonds with the Cl atoms. Five covalent bonds require the sharing of 10 electrons. It is impossible for phosphorus to obey the octet rule. The P atom can use s and p orbitals for four of the bonds. The remaining bond will involve a 3d orbital of phosphorus. Being in the third period, phosphorus has d orbitals available for bonding when needed. Elements in the third row of the periodic table and lower that utilize 3d orbitals for bonding are said to have expanded octets.
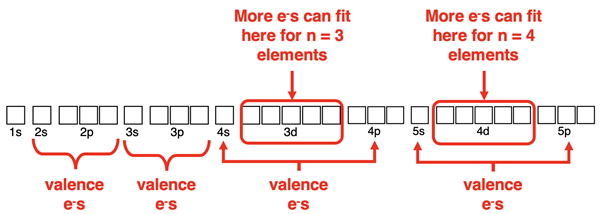
Drawing Electron Dot Diagrams
 Previously in Lesson 2 we learned a method for drawing Lewis electron dot diagrams. The method involved determining the number of valence shell electrons in a molecule, drawing its skeleton structure, and then populating all atoms with an octet of electrons. The fourth and most important step involved counting the number of electrons used in the diagram and comparing it to the number of valence shell electrons. If the number of electrons used is less than the number of valence electrons, then adjustments must be made. The adjustment involves adding the additional valence electrons to the central atom.
Previously in Lesson 2 we learned a method for drawing Lewis electron dot diagrams. The method involved determining the number of valence shell electrons in a molecule, drawing its skeleton structure, and then populating all atoms with an octet of electrons. The fourth and most important step involved counting the number of electrons used in the diagram and comparing it to the number of valence shell electrons. If the number of electrons used is less than the number of valence electrons, then adjustments must be made. The adjustment involves adding the additional valence electrons to the central atom.
Row 3 elements and beyond can exceed their octet by using d orbitals for bonding. This will never happen with n = 2 nonmetals like carbon, nitrogen, oxygen, and fluorine since there are no d orbitals at the n=2 energy level. But the atoms of row 3 elements and lower on the periodic table are able to hold more than 8 electrons when needed.
The examples on this page demonstrate this method being practiced for the so-called octet breakers. Follow along with the process. Be sure to try several on your own. There are three practice diagrams in the Check Your Understanding section.
Example: SF4 Molecule
Steps 1-3 and the start of Step 4 of our method are shown below:
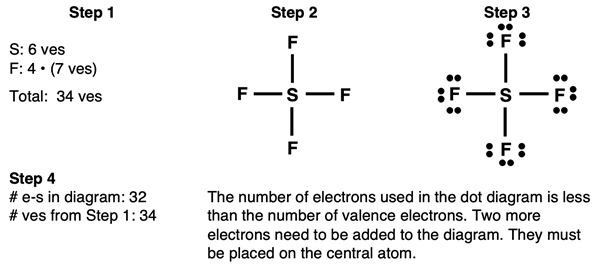
 The last electron pair can be placed anywhere on the central atom. You can keep the bonds (shared pair) as they are and add the final pair between the bonds.
The last electron pair can be placed anywhere on the central atom. You can keep the bonds (shared pair) as they are and add the final pair between the bonds.
Do the final check by answering these two questions:
- Other than the central atom, do all other atoms have an octet of electrons?
- Is the number of electrons shown in the diagram equal to the number of valence electrons in the molecule?
The answers to both these questions is yes. We can be confident in our diagram.
Example: PF5 Molecule
Steps 1-3 and the start of Step 4 of our method are shown below:
This example is an obvious case of an octet breaker since the central atom has five electron pairs involved in bonding. Once step 4 has been completed, it is obvious that the two requirements have been met. Terminal atoms all have an octet of electrons. And the number of electrons in the diagram is equal to the number of valence electrons.
Example: XeF4 Molecule
Steps 1-3 and the start of Step 4 of our method are shown below:
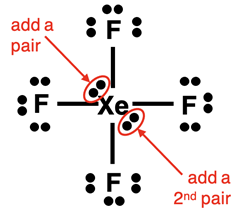
The final two electron pairs can be placed anywhere around the central atom. Simply squeeze them in between the bonds. Then do your final check to make sure there are Yes! answers to the following two questions:
- Other than the central atom, do all other atoms have an octet of electrons?
- Is the number of electrons shown in the diagram equal to the number of valence electrons in the molecule?
Example: I3- ion
Our first example of an ion is I
3-. Electron dot diagrams for ions are done the same as for neutral molecules. The only change has to do with the valence electron count. A 1- ion like I
3- has one more electron than protons. An electron will have to be added for the 1- charge.
Steps 1-3 and the start of Step 4 of our method are shown below:
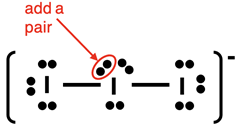
The final electron pair can be added anywhere to the central atom. We slide one of the lone pairs to the side and squeeze the added pair next to it.
Once again, make sure you do the check:
- Other than the central atom, do all other atoms have an octet of electrons?
- Is the number of electrons shown in the diagram equal to the number of valence electrons in the molecule?
The answer to both questions is Yes! The diagram is complete.
Example: ClF3 Molecule
Steps 1-3 and the start of Step 4 of our method are shown below:
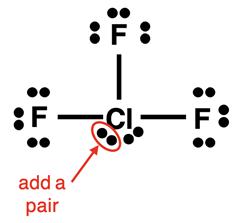
The final electron pair can be added anywhere to the central atom. We slide the lone pair below the Cl atom to the side and squeeze the added pair next to it.
Finally, do the check:
- Other than the central atom, do all other atoms have an octet of electrons?
- Is the number of electrons shown in the diagram equal to the number of valence electrons in the molecule?
The answer to both questions is Yes! The diagram is complete.
Boron Compounds
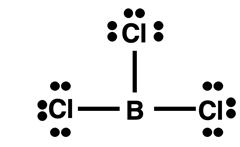
This page has been all about octet breakers - molecules that contain a central atom that breaks the octet rule. All examples so far have included central atoms with expanded octets. Now we will look at boron. Boron (
5B) is a metalloid in the second period. A boron atom has only three valence shell electrons. It most commonly forms covalent bonds with nonmetals but it does not satisfy the octet rule. Boron will help the atom that it bonds with to satisfy its octet rule. But boron itself usually falls short of a filled octet. This is shown in the Lewis diagram at the right for BCl
3. The moral of the story is that if you only bring three valence electrons to the party, then it is a bit unreasonable to expect to do some electron sharing and end up with eight valence electrons.
Beryllium Compounds
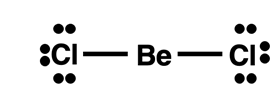
Beryllium is a metal in the second period. We expect metals to participate in ionic bonding with nonmetals. However, most beryllium compounds form bonds with nonmetals that have a very high covalent character. The small size of the atom with its densely packed charge allows beryllium to draw very near to the nonmetal and attract its electrons. Rather than transferring an electron to the nonmetal, beryllium tends to share electrons and participate in covalent bonding. However, beryllium brings only two valence shell electrons to the party and as such is not going to achieve an octet by sharing. Its more significant role to molecular stability is to share its electrons in order to help the nonmetal achieve a stable octet. Expect beryllium to fall far short of obtaining eight valence electrons.
Before You Leave
- Download our Study Card on Expanded Octets. Save it to a safe location and use it as a review tool.
- Practice! Try our Concept Builder titled Electron Dot Structures.
- The Check Your Understanding section below include questions with answers and explanations. It provides a great chance to self-assess your understanding.
Check Your Understanding
Use the following questions to assess your understanding. Tap the Check Answer buttons when ready.
1. Construct a Lewis electron dot diagram for the SF6 molecule.
2. Construct a Lewis electron dot diagram for the ICl
2- ion.
3. Construct a Lewis electron dot diagram for the ICl
4- ion.
4. Phosphorus and nitrogen are in the same elemental family – group 15. You would expect very similar chemical properties of these two elements. For instance, both form similar compounds with chlorine – NCl
3 and PCl
3. But phosphorus also forms PCl
5 while nitrogen does not form NCl
5. Use this lesson to explain why.