Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 2: Classifying Chemical Reactions
Part d: Double Replacement Reactions
Part a:
Decomposition and Synthesis Reactions
Part b:
Combustion Reactions
Part c:
Single Replacement Reactions
Part d: Double Replacement Reactions
Part e:
Predicting Products
Reaction Types and Predicting Products
Lesson 2 has focused on using a knowledge of reaction types to predict the products of a reaction. Five reaction types were identified. Synthesis (a.k.a., combination) and decomposition reactions were discussed in Lesson 2a. Combustion reactions were discussed in Lesson 2b. Single replacement reactions were discussed in Lesson 2c. Several examples were used to demonstrate how a knowledge of these types of reactions could be used to predict products and construct balanced chemical equations. In Lesson 2d, we will discuss a fifth reaction type category - double replacement reactions. We will take the discussion a bit further as we develop a model regarding ionic solids dissolved in water.
What is a Double Replacement Reaction?
A double replacement reaction is a reaction in which a cation (positive ion) in an ionic compound replaces another cation in a second ionic compound. You can think of the two cations as switching places or trading anion partners. This type of reaction is also referred to as a double displacement reaction. The generic form of a double replacement reaction is
AB + CD → CB + AD
There are two ionic compounds on the reactant side – AB and CD. The cations are A and C. The anions are B and D. The cation A is initially in an ionic compound with anion B. Cation C is in an ionic compound with anion D. After the reaction, cation A is with anion D and cation C is with anion B. Cations A and C have switched places. They have replaced each other or changed places with each other to form new ionic compounds.
A specific example of a double replacement reaction is the reaction of sodium chloride (NaCl) with silver nitrate (AgNO3).
NaCl(aq) + AgNO3(aq) → AgCl(s) + NaNO3(aq)
AB + CD → CB + AD

The state symbols in the above equation reveal much about the nature of a double replacement reaction. First, both reactants are ionic compounds dissolved in water – (aq). Double replacement reactions occur in aqueous solutions. Second, one of the products is an ionic compound dissolved in water and the other is an ionic compound in the solid state. The AgCl(s) is a precipitate. Double replacement reactions result in the formation of a precipitate.
Here are two more examples of balanced chemical equations for double replacement reactions.
Na2SO4(aq) + BaCl2(aq) → BaSO4(s) + 2 NaCl(aq)
2 NH4OH(aq) + CuCl2(aq) → Cu(OH)2(s) + 2 NH4Cl(aq)

Observe that in each case there are two ionic compounds as reactants and two ionic compounds as products. Observe that both reactants are ionic compounds dissolved in water. And observe that one product is an ionic compound dissolved in water and the other product is an insoluble ionic compound (solid state).
Understanding Solubility
Some ionic compounds dissolve in water. These are referred to as soluble compounds. Other ionic compounds are insoluble compounds; the degree to which they dissolve in water is so minimal that they can be said to not dissolve. Whether or not an ionic compound is soluble or insoluble depends on what ions are in the compound. Experimental studies of numerous compounds have led to rules for predicting the solubility of compounds in water. The rules are known as solubility rules.
A list of solubility rules is shown below. The rules can be used to predict if an ionic compound will dissolve in water. If a compound dissolves, it will be in the (aq) state. If it does not dissolve when dumped in water, it will remain in the solid state – (s) state. Observe that there are exceptions to many of the rules.
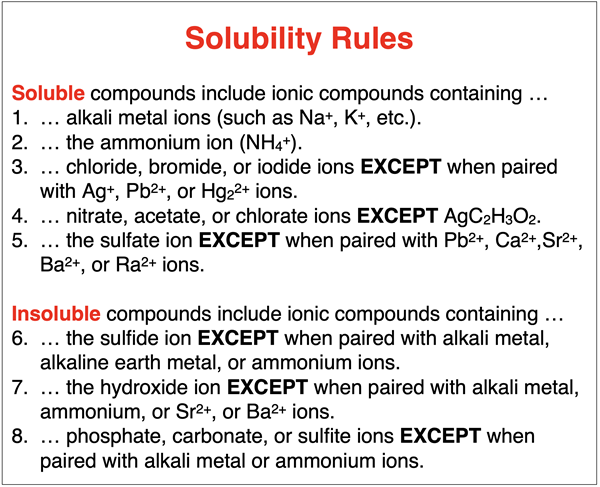
The examples below demonstrate how to use the rules to determine the solubility and insolubility of a variety of ionic compounds.

Understanding Aqueous Solutions
When you see the NaCl(aq) designation, you know it is referring to the ionic compound dissolved in water. Ionic compounds that are dissolved in water dissociate or split apart into ions. An aqueous solution of NaCl does not contain any units of NaCl. Instead, the solution form of NaCl contains sodium ions (Na+) and chloride ions (Cl-). With very rare exceptions, the same is true of any ionic compound dissolved in water. We will certainly have more to say about this in our Solutions unit. But for now, begin building the mental model that NaCl(aq) refers to positive and negative ions dispersed throughout the solution.
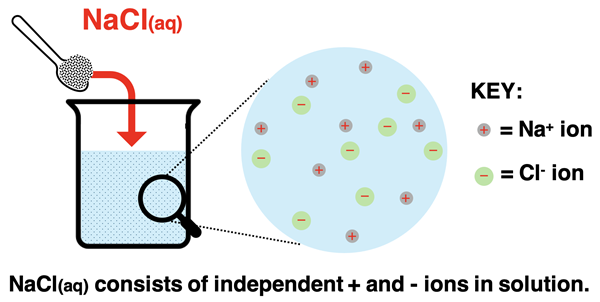
The ions that are in solution can always be determined from the molecular formula of the ionic compound. The formula indicates the cation and the anion by its symbol. The relative numbers of each are indicated by the subscripts (assumed 1 if not shown). And the charges of the ions can be determined in a variety of ways. These ways include the location of the element on the periodic table (for main group elements), a polyatomic ion list (for polyatomic ions), and logical reasoning (for transition metals). This has all been discussed elsewhere in our Chemistry Tutorial. Here are a few examples for review:

Predicting the Precipitate
Now it is time to put a lot of our understandings together to predict the precipitate in a double replacement reaction. Here are the understandings that you’ll need to put together:
- Double Replacement Reactions: a) Cations replace one another or trade places. b) Both reactants are ionic compounds dissolved in water. c) One product is an ionic compound dissolved in water. The second product is an insoluble ionic compound (solid state).
- Aqueous Solutions: a) Ionic compounds exist as ions when in the aqueous state. b) The ions and their charges can be determined from the molecular formula or name.
- Solubility: Solubility rules can be used to determine if an ionic compound is soluble or insoluble.
As needed, review the discussion above to gain a firm understanding of these concepts. The discussion that follows is dependent upon these understandings.
Example 1:
Consider the double replacement reaction between aqueous solutions of copper(II) chloride and potassium hydroxide. Determine the precipitate and write the balanced chemical equation.
Solution:
We will start off the process by identifying the reactant formulae and the reactant side of the skeleton equation. The reactants are CuCl
2(aq) and KOH(aq). The reactant side of the skeleton equation is
CuCl2(aq) + KOH(aq) → Products???
This situation can be pictured as follows:
The products will be the result of the cations replacing each other. If K
+ and Cu
2+ replace each other, the product formulae would be KCl and Cu(OH)
2. We need to use solubility rules to determine their state – (aq) if soluble and (s) if insoluble. Both
rule 1 and rule 3 would indicate that KCl is soluble and exists as KCl(aq).
Rule 7 indicates that Cu(OH)
2 is insoluble and exists as Cu(OH)
2(s). Copper hydroxide is the precipitate in this reaction and K
+ and Cl
- ions remain dissolved in solution. Our picture is complete:
The skeleton equation is:
CuCl2(aq) + KOH(aq) → KCl(aq) + Cu(OH)2(s)
Coefficients can be inserted in front of the formulae to balance the chemical equation.
CuCl2(aq) + 2 KOH(aq) → 2 KCl(aq) + Cu(OH)2(s)
Procedure for Predicting the Precipitate
The procedure used in
Example 1 to predict the precipitate and write the balanced chemical equation was:
- Determine the reactant formulae (if not given).
- Determine the ions initially present in the solution. These are the ions the two reactants are composed of.
- Identify the formulae of the two products. This involves switching the cations so that they are with different anion.
- Use solubility rules to determine which, if any, of the two products are insoluble. Any that is insoluble will precipitate as a solid. Those that are soluble exists in the aqueous state.
- Use the reactant formulae (step 1) and the product formulae (step 3) and state (step 4) to write the skeleton equation.
- Insert coefficients in front of formulae to write the balanced chemical equation.
The pictures were helpful to the analysis and we recommend using them. They will be even more helpful in later sections when we learn to write complete ionic equations and net ionic equations. Before we learn that, let’s practice a few more:
Example 2
Aqueous solutions of lead(II) nitrate and sodium chloride are mixed. Determine the precipitate and write the balanced chemical equation.
Solution:
The reactant formulae are Pb(NO
3)
2(aq) and NaCl(aq). The four ions in solution are Pb
2+, NO
3-, Na
+, and Cl
-. For a precipitate to form, the cations must trade locations and one or both of the products must be insoluble. The products are NaNO
3 and PbCl
2. Now check
the solubility rules to see if either is insoluble and thus a precipitate. The NaNO
3 is soluble (
Rules 1 and 4) and exists in the aqueous state as NaNO
3(aq). But the PbCl
2 is insoluble (the exception of
Rule 3) and will precipitate as PbCl
2(s). This situation can be pictured as …
The product formulae are NaNO
3(aq) and PbCl
2(s). The skeleton equation is …
Pb(NO3)2(aq) + NaCl(aq) → NaNO3(aq) + PbCl2(s)
The balanced chemical equation can be written by inserting coefficients in front of the formulae.
Pb(NO3)2(aq) + 2 NaCl(aq) → 2 NaNO3(aq) + PbCl2(s)
Example 3
Aqueous solutions of barium chloride and sodium sulfate are mixed. Determine the precipitate and write the balanced chemical equation.
Solution:
The reactant formulae are BaCl
2(aq) and Na
2SO
4(aq). The four ions in solution are Ba
2+, Cl
-, Na
+, and SO
42-. For a precipitate to form, the cations must trade locations and one or both of the products must be insoluble. The products are BaSO
4 and NaCl. Now check the
solubility rules to see if either is insoluble and thus a precipitate. The NaCl is soluble (
Rules 1 and 3) and exists in the aqueous state as NaCl(aq). But the BaSO
4 is insoluble (the exception of
Rule 5) and will precipitate as BaSO
4(s). This situation can be pictured as …
The product formulae are NaCl(aq) and BaSO
4(s). The skeleton equation is …
BaCl2(aq) + Na2SO4(aq) → NaCl(aq) + BaSO4(s)
The balanced chemical equation can be written by inserting coefficients in front of the formulae.
BaCl2(aq) + Na2SO4(aq) → 2 NaCl(aq) + BaSO4(s)
Complete Ionic Equations and Net Ionic Equations
Much of the emphasis in the approach above has been to interpret the system in terms of ions. This makes sense because ionic compounds in aqueous solutions exist as dissolved ions and not as molecules. But it is perhaps strange to do a complete ion analysis and then turn around and write the reaction by listing the molecules involved. In
Example 1, the balanced chemical equation was …
CuCl2(aq) + 2 KOH(aq) → Cu(OH)2(s) + 2 KCl(aq)
All formulae in the equation infer a molecule despite the fact that the only molecule involved is the solid precipitate. So, let’s re-think
Example 1 and write the equation in terms of ions. You may recall our picture of the situation:
A beaker with Cu
2+ and Cl
- ions is added to a beaker with K
+ and OH
- ions. The Cu
2+ ions from one beaker get together with the OH
- ions from the other beaker and form a solid precipitate – Cu(OH)
2. The K
+ and the Cl
- remain in solution. Now let’s describe this completely as an ionic equation. We will begin by writing the skeleton equation (formulae only, no coefficients, not balanced):
Cu2+(aq) + Cl-(aq) + K+(aq) + OH-(aq) → K+(aq) + Cl-(aq) + Cu(OH)2(s)
Now we will insert coefficients in front of the formulae to balance both the atoms and the charges:
Cu2+(aq) + 2 Cl-(aq) + 2 K+(aq) + 2 OH-(aq) → 2 K+(aq) + 2 Cl-(aq) + Cu(OH)2(s)

The above equation has a particular name. It is referred to as the
complete ionic equation. Let’s look at this equation from the context of our original description of double replacement reactions. We stated three important characteristics. They are listed at the right. Observe that there are two dissolved ionic compounds on the reactant side. Being dissolved, they exist as ions. And there is one dissolved ionic compound on the product side, also existing as ions. And finally, there is an ionic compound on the product side that is insoluble and thus existing in the solid state.

A further analysis of our complete ionic equation reveals something strange about two of our ions – Cl
- and K
+ ions. They never changed. They were present in the beaker before the reaction and present in the beaker after the reaction. They were not involved in the reaction. We refer to them as
spectator ions. Like spectators at a sporting event, they are not involved in the game. If we eliminate them from the complete ionic equation, then we end up with two ions on the reactant side and one insoluble solid on the product side. This is shown below. When the complete ionic equation is re-written with the spectator ions removed, we have a new equation that we refer to as the
net ionic equation.
Let’s repeat the same type of ion analysis of
Example 2 to generate a complete ionic equation and a net ionic equation. The balanced chemical equation that we wrote was
Pb(NO3)2(aq) + 2 NaCl(aq) → 2 NaNO3(aq) + PbCl2(s)
And our picture of the situation is
We can describe this as a beaker with Pb
2+ and NO
3- ions is added to a beaker with Na
+ and Cl
- ions. The Pb
2+ ions from one beaker get together with the Cl
- ions from the other beaker to form a solid precipitate – PbCl
2. The Na
+ and the NO
3- remain in the solution. They are the spectator ions. They do not get involved in the reaction. The complete ionic equation can be written and balanced as
The net ionic equation can be written by removing the spectator ions from the complete ionic equation. The result is
Three Equations for One Event
Double replacement reactions are described by three different equations – a molecular equation, a complete ionic equation, and a net ionic equation. The
molecular equation shows the reactant and the product formulae as molecules. But we know that molecules are not involved in the reaction. It is the ions that are involved. The
complete ionic equation shows the same system but represents the reactants and products as they actually exist – as ions or as a solid precipitate. The complete ionic equation includes two spectator ions on the reactant and the product side. The
net ionic equation can be thought of as the complete ionic equation with the spectator ions removed. It shows the actual action that occurs – two ions coming together to form an insoluble precipitate.
Can There Ever Be No Reaction?
Is it possible that two ionic solids dissolve in water and no precipitate is formed? Yes. In fact, it is quite common. Suppose the cation from compound #1 and the anion from compound #2 are soluble in water. And suppose the cation from compound #2 and the anion from compound #1 are also soluble in water. If this is the case, then we would say that no reaction occurs. Here’s an example.
NaCl(aq) + KOH(aq) → KCl(aq) + NaOH(aq)
Neither of the products are insoluble. The complete ionic equation for this would display four spectator ions. That is, there are four ions that do not get involved in a reaction. Neither KCl nor NaOH forms a precipitate.
Na+(aq) + Cl-(aq) + K+(aq) + OH- (aq) → K+(aq) + Cl-(aq) + Na+(aq) + OH-(aq)
If none of the ions are getting involved in the action, the only conclusion that could be made is that there is no action. A reaction does not occur.
Before You Leave
- Download our Study Card on Types of Reactions. (It covers Lessons 2a, 2b, 2c, and some of Lesson 2d.) Save it to a safe location and use it as a review tool.
- Download our Study Card on Precipitation Reactions. Save it to a safe location and use it as a review tool.
- Once you have some comfort with Lessons 2a through 2d, try our Precipitation Reactions Concept Builder. It will provide great practice with using solubility rules, identifying precipitates, and writing net ionic equations.
- The Check Your Understanding section below includes questions with answers and explanations. It provides a great chance to self-assess your understanding.
Check Your Understanding
Use the following questions to assess your understanding. Tap the Check Answer buttons when ready.
1. For double replacement reactions, …
- How many reactants are there?
- How many products are there?
- Are the reactants and products isolated elements, compounds, or both?
- What is the state symbol for the reactants and the products?
2. The following chemical equations list the reactants only. In which cases could the reaction be a double replacement reaction? Select all that apply.
Reaction A: N
2(g) + H
2(g)
→
Reaction B: Al(OH)
3(aq) + CuCl
2(aq)
→
Reaction C: NH
4NO
3(s)
→
Reaction D: Na
2CO
3(aq) + MgCl
2(aq)
→
Reaction E: CH
4(g) + O
2(g)
→
Reaction F: F
2(aq) + CuI
2(aq)
→
3. Given the following descriptions of a double replacement reaction, write the skeleton equation and the balanced chemical equation (a.k.a., the molecular equation):
- Copper(II) acetate is precipitated from the reaction of aqueous solutions of copper(II) chloride and sodium acetate.
- When aqueous solutions of potassium hydroxide and iron(III) chloride are mixed, iron(III) hydroxide is precipitated from the solution.
- Lead(II) sulfate precipitate is formed when aqueous solutions of lead(II) nitrate and sodium sulfate are reacted.
4. For the following compounds, identify whether they are soluble or insoluble in water. Identify the solubility rule (by number) that you used to make your decision.
- sodium sulfide
- iron(II) sulfide
- ammonium acetate
- magnesium sulfate
- aluminum hydroxide
- calcium carbonate
5. Consider the following diagrams depicting two solids to be dissolved in water. Once mixed, a precipitate may or may not be formed. For each case, identify if there is a precipitate. If so, identify the formula of the precipitate. Finally, identify which ions remain in the solution.
6. For the following reactions, write the molecular equation and the net ionic equation. If there is no reaction, state “NR” and don’t bother with any of the equations.
- Na2CO3(aq) + MgCl2(aq) → ???
- K2SO3(aq) + MgCl2(aq) → ???
- NaOH(aq) + Ca(NO3)2(aq) → ???
- (NH4)2S(aq) + FeCl3(aq) → ???