Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 2: Classifying Chemical Reactions
Part c: Single Replacement Reactions
Part a:
Decomposition and Synthesis Reactions
Part b:
Combustion Reactions
Part c: Single Replacement Reactions
Part d:
Double Replacement Reactions
Part e:
Predicting Products
Reaction Types and Predicting Products
Lesson 2 began with a discussion of reaction types. The emphasis was on using a knowledge of reaction types to predict the products of a reaction. Five reaction types were identified. Synthesis (a.k.a., combination) and decomposition reactions were discussed in Lesson 2a and combustion reactions were discussed in Lesson 2b. Several examples were used to demonstrate how a knowledge of these types of reactions could be used to predict products and construct balanced chemical equations. In Lesson 2c, we will discuss a fourth reaction type category - single replacement reactions.
What is a Single Replacement Reaction?
A single replacement reaction is a reaction in which one element replaces another element in a compound. This type of reaction is also referred to as a single displacement reaction. The generic form of a single replacement reaction is
A + BC → B + AC
In this reaction the element A replaces the element B in the compound BC. The element A starts as a free element and finishes as the positive ion of an ionic compound. The element B starts as the positive ion in an ionic compound and finishes as a free element. Element A has replaced element B.
 Cation Replacement
Cation Replacement
Single replacement reactions commonly occur when the solid metal (A) is placed in an aqueous solution of the ionic compound (BC). You can expect that a metal will replace another metallic ion in the solution. The metallic element A becomes an ion, and the metallic ion B becomes a solid.
Suppose that a copper wire is immersed in an aqueous solution of silver(I) nitrate. The copper replaces the silver ions. Put another way, the copper and the silver trade places. The balanced chemical equation for the reaction is …
Cu(s) + 2 AgNO3(aq) → 2 Ag(s) + Cu(NO3)2(aq)
A + BC → B + AC
When this reaction is performed in the lab, a solid copper wire is typically shaped into a coil. It is then placed in a colorless solution of AgNO3. Over the course of time, grey flakes of silver metal can be observed forming on the surface of the copper wire. The solution gradually turns a blue color, indicated the presence of Cu(NO3)2. The formation of a solid and a color change are observed as evidence of a chemical reaction.
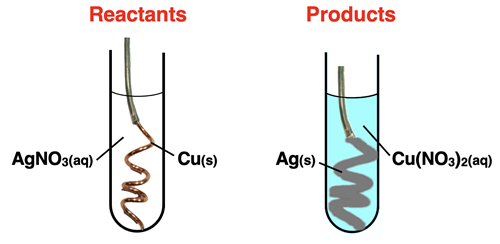
As a second example of a single replacement reaction, consider the reaction of sodium metal in water. The balanced chemical equation is …
2 Na(s) + 2 H2O(l) → H2(g) + 2 NaOH(aq)
 The water reactant can be thought of as H•OH. The element Na replaces the element H of H•OH. Put another way, Na and H trade places. When this reaction is observed in a lab (with CAUTION … and with a small amount of Na), bubbles are formed and a large amount of heat (and even flames) can be observed. This is evidence of a chemical reaction.
The water reactant can be thought of as H•OH. The element Na replaces the element H of H•OH. Put another way, Na and H trade places. When this reaction is observed in a lab (with CAUTION … and with a small amount of Na), bubbles are formed and a large amount of heat (and even flames) can be observed. This is evidence of a chemical reaction.
The reaction of sodium with water is very dangerous. Reaction of large quantities of sodium can generate explosive amounts of heat, ultimately expelling reactive sodium into the air and onto innocent bystanders. The safest means of observing the reaction is by watching a YouTube video, thus placing time, distance, and a screen between you and the reaction.
Image Source
Reactivity Series
Not every metallic element will replace a metal ion in a dissolved compound. Metals are ordered according to their degree of reactivity. Only a more reactive metal will replace a metal ion in a compound. The chart below is known as a Metal Reactivity Series.
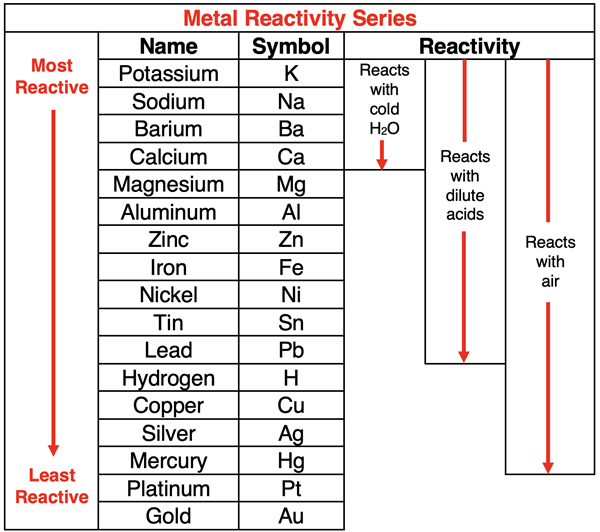
Metal elements higher on the chart will react with ionic compounds containing metal ions located lower on the chart. So, zinc (Zn) would replace silver ions (Ag+) in AgNO3(aq). However, silver would not replace zinc ions in Zn(NO3)2(aq).
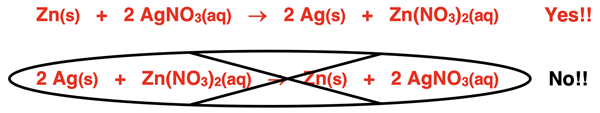
An understanding of the metal reactivity series will assist in predicting whether a single replacement reaction will take place.
Anion Replacement
Metals are not the only elements that can participate in single replacement reactions Nonmetal halogens are also observed to react with ionic compounds containing a less reactive halogen. Just as metals replace metals, you can expect a halogen to replace another halogen. As an example, bromine will react with an aqueous solution of sodium iodide. The balanced chemical equation is …
Br2(aq) + 2 NaI(aq) → I2(aq) + 2 NaBr(aq)
 NOTE: in their elemental state, both bromine and iodine are diatomic elements (HONClBrIF).
NOTE: in their elemental state, both bromine and iodine are diatomic elements (HONClBrIF).
Predicting whether a reaction occurs requires that attention be given to the relative reactivity of the two halogens. The chart at the right is the reactivity series for halogens. Elements highest on the chart are the most reactive. So, while bromine can replace iodine as shown by the above equation, iodine is not reactive enough to replace bromine.
I2(aq) + NaBr(aq) → No Reaction
Predicting Products and Writing Balanced Equations
 A common task required in introductory Chemistry courses is to write balanced chemical equations for single replacement reactions. The first step is to use a reactivity series to decide if a reaction actually takes place. An element higher on the chart will react with an ion located lower on the chart. The second step is to identify the reactant and product formulae. If the reactant element is a metal, then it replaces the cation in the ionic compound. (Cations are listed first in the formulae of ionic compounds.) If the reactant element is a nonmetal, then it will replace the anion of the ionic compound. The third step involves writing a skeleton equation for the single replacement reaction. The fourth step involves adding coefficients to balance the chemical equation.
A common task required in introductory Chemistry courses is to write balanced chemical equations for single replacement reactions. The first step is to use a reactivity series to decide if a reaction actually takes place. An element higher on the chart will react with an ion located lower on the chart. The second step is to identify the reactant and product formulae. If the reactant element is a metal, then it replaces the cation in the ionic compound. (Cations are listed first in the formulae of ionic compounds.) If the reactant element is a nonmetal, then it will replace the anion of the ionic compound. The third step involves writing a skeleton equation for the single replacement reaction. The fourth step involves adding coefficients to balance the chemical equation.
We have provided four examples below to demonstrate the use of the above method and logic. Take time to try them yourself. Tap the Check Answer button to view the answer and complete solution.
Example 1
Aluminum metal is added to a solution of tin(IV) chloride. Write the balanced chemical equation for the reaction.
Example 2
Chlorine gas is bubbled into an aqueous solution of sodium iodide. Write the balanced chemical equation for the reaction.
Example 3
Calcium metal is added to a beaker of water. Write the balanced chemical equation for the reaction of calcium and water.
Example 4
Copper metal is added to an aqueous solution of iron(II) nitrate. Write the balanced chemical equation for the reaction.
Before You Leave
- Download our Study Card on Types of Reactions. (It covers Lessons 2a, 2b, 2c, and some of Lesson 2d.) Save it to a safe location and use it as a review tool.
- Once you have some comfort with Lessons 2a through 2d, try our Chemical Reaction Type Concept Builder. It will provide awesome practice on all five reaction types.
- The Check Your Understanding section below includes questions with answers and explanations. It provides a great chance to self-assess your understanding.
Check Your Understanding
Use the following questions to assess your understanding. Tap the Check Answer buttons when ready.
1. REVIEW: How can you distinguish between the cation and the anion in an ionic formula?
2. When writing a chemical equation for a single replacement reaction, you must be able to write the formula for an ionic compound. Explain the rules or procedure for doing this.
3. Can a metal ever replace a nonmetal in a single replacement reaction?
4. The following equations list the reactants only. In which cases could the reaction be a single replacement reaction? Select all that apply.
Reaction A: N
2(g) + H
2(g)
→
Reaction B: Al(s) + CuCl
2(aq)
→
Reaction C: NH
4NO
3(s)
→
Reaction D: CH
4(g) + O
2(g)
→
Reaction E: F
2(aq) + CuI
2(aq)
→
5. For each of the following, identify if a reaction occurs. For each that does occur, identify product formulae, write the skeleton equation, and insert coefficients to balance the chemical equation.
- Al(s) + CuI2(aq) →
- F2(aq) + CuBr2(aq) →
- Pb(s) + ZnCl2(aq) →
- Mg(s) + PbCl2(aq) →
- Sn(s) + CaCl2(aq) →
- Br2(aq) + NaCl(aq) →