Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 1: Kinetics of Reactions
Part e: Reaction Mechanisms
Part a:
Reaction Rates
Part b:
Factors Affecting Reaction Rates
Part c:
The Collision Model of Reactions
Part d:
Rate Equations
Part e: Reaction Mechanisms
What is a Reaction Mechanism?
Consider the synthesis of ammonia:
N2(g) + 3 H2(g) → 2 NH3(g)
If you think about this reaction in terms of the Collision Model, you might think that ammonia is produced when one molecule of nitrogen and three molecules of hydrogen simultaneously collide with sufficient energy and the proper orientation. The odds of this ever happening are slim to none. It is rare that three particles would ever collide simultaneously, let along four particles … with sufficient energy … and the proper orientation. What is going on? How is ammonia ever produced by this reaction? The answer: in steps.

 A reaction mechanism represents an effort to explain the step-by-step details of how a reaction occurs. It expresses the belief that reactions take place in a series of steps known as elementary steps. Each elementary step is a reaction involving a small number of particles. The collective sum of these steps is equivalent to the overall chemical reaction. While the balanced chemical equation indicates what reacts and what is produced, the reaction mechanism provides the play-by-play story of the reaction. The balanced chemical equation shows what happens; but the reaction mechanism shows how it happens.
A reaction mechanism represents an effort to explain the step-by-step details of how a reaction occurs. It expresses the belief that reactions take place in a series of steps known as elementary steps. Each elementary step is a reaction involving a small number of particles. The collective sum of these steps is equivalent to the overall chemical reaction. While the balanced chemical equation indicates what reacts and what is produced, the reaction mechanism provides the play-by-play story of the reaction. The balanced chemical equation shows what happens; but the reaction mechanism shows how it happens.
Elementary Steps and Intermediates
Let’s consider the reaction between nitrogen dioxide and carbon monoxide:
NO2(g) + CO(g) → NO(g) + CO2(g)
One of the possible mechanisms for this reaction includes this simple two-step process
Step 1: NO2 + NO2 → NO3 + NO
Step 2: NO3 + CO → NO2 + CO2
Step 1 and step 2 are the elementary steps of this proposed mechanism. If you look carefully, you will notice a particle present in the mechanism that isn’t in the balanced chemical equation. The NO3 molecule is produced in step 1 and then reacted away in step 2. In this mechanism, NO3 is acting as an intermediate. An intermediate is a short-lived particle that is a product in an early step of a mechanism and a reactant in a later step. While it shows up in the elementary steps, it is neither a reactant nor a product in the balanced chemical equation.
It was mentioned earlier that the overall chemical reaction is the collective sum of the elementary steps. That is to say that if we add all the formulae for all the reactants and all the products (much in the same way as we add terms when adding algebraic equations) and simplify (as we do in algebra), we should end up with the balanced chemical equation. For the above mechanism, the collective sum of all the formulae is …
NO2 + NO2 + NO3 + CO → NO3 + NO + NO2 + CO2
We can perform the first simplification step by cancelling formulae that show up on both the left and the right side of the reaction arrow. This includes the intermediate NO3 (as is always expected). It also includes one NO2 molecule.
NO2 + NO2 + NO3 + CO → NO3 + NO + NO2 + CO2
After cancelling formulae, the equation becomes:
NO2 + CO → NO + CO2
The result of the addition and simplification is that we have the balanced chemical equation. For a proposed mechanism to be valid, the collective sum of its elementary steps must add up to the balanced chemical equation for the reaction.
Mechanisms and Rate Equations
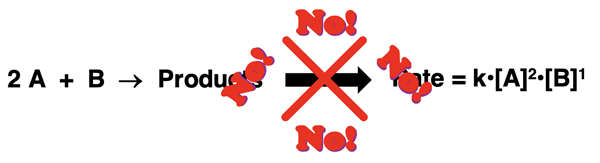
As discussed in Lesson 1d, rate equations are determined experimentally using a method like the method of initial rates. The rate equation has nothing to do with the balanced chemical equation and its coefficients. If the exponent on a reactant concentration matches the coefficient on that reactant, then consider it to be a coincidence and not a rule. Balanced chemical equations for a reaction are not the tool used to derive a rate equation. An experimental study is the means of determining a rate equation.
That being said, it is possible to generate a rate equation from an elementary step in a mechanism. To do so, you must first be able to identify which of the steps is the rate determining step. The rate determining step is the slowest step in the multi-step process. The reaction rate depends upon the rate of that particular step. We have added some information to the previously discussed mechanism. The relative rate of the two elementary steps is shown to the left of the chemical equation.
Step 1: NO2 + NO2 → NO3 + NO Slow
Step 2: NO3 + CO → NO2 + CO2 Fast
Step 1 is the rate-determining step. The reaction rate will be based on this step. In general, the rate at which an elementary step takes place has the following form:
Rate = k•[Rxt #1]x•[Rxt #2]y
(for elementary steps only)
The exponents x and y are the coefficients in the equation for the elementary step. This mechanism would predict the rate equation to be
Rate = k•[NO2]2
Just because you can write a rate equation from a mechanism, does not mean that the equation describes the rate for the reaction under study. All it means is that the mechanism predicts the reaction to have that particular rate equation. If the experimentally derived rate equation does not match the equation predicted by the mechanism, then the mechanism would be considered invalid.
Validating a Mechanism
 A proposed mechanism is an educated guess regarding how a reaction takes place. Not all proposed mechanisms are created equal. A valid mechanism will satisfy two requirements.
A proposed mechanism is an educated guess regarding how a reaction takes place. Not all proposed mechanisms are created equal. A valid mechanism will satisfy two requirements.
- The collective sum of the elementary steps add up to the balanced chemical equation for the reaction.
- The rate equation proposed by a mechanism matches the experimentally derived rate equation.
If a proposed mechanism fails either one of these steps, then it is considered invalid and no longer regarded as the possible mechanism.
A proposed mechanism can never be proven to be the
correct mechanism. There is often more than one plausible mechanism that satisfies the two requirements. Evidence and reasoning can often be presented in favor of one over the other(s). Here are some considerations that are used to support a mechanism.
- Elementary steps typically involve one, two, or three reactant particles. We refer to this number as the molecularity. Elementary steps with a molecularity of 3 or higher are less likely to occur than steps with a molecularity of 1 or 2. Mechanisms with an elementary step involving three or more particles is less likely to be valid.
- Most mechanisms have intermediates. These are short-lived substances that can often be detected experimentally. Detection of an intermediate that is present in a proposed mechanism lends support for that mechanism. However, not being able to detect a mechanism does not disprove the mechanism.
Another Mechanism Example
Hydrogen peroxide slowly
decomposes into water and oxygen gas. The balanced chemical equation is
2 H2O2(aq) → 2 H2O(l) + O2(g)
The reaction is thought to proceed by the following two-step mechanism:
Step 1: H
2O
2 → H
2O + O Slow
Step 2: O + H
2O
2 → H
2O + O
2 Fast
The intermediate in this mechanism is monatomic O. It is formed in the first step and reacts away in the second step. If the two elementary steps are added together, the result is:
H2O2 + O + H2O2 → H2O + O + H2O + O2
The intermediate - monatomic O - can be cancelled from each side of the equation.
H2O2 + H2O2 → H2O + H2O + O2
Then, like terms can be grouped to produce the balanced chemical equation.
2 H2O2 → 2 H2O + O2
Step 1 is the slow step and thus the rate-determining step. The rate equation would be based on this step. This mechanism would propose that
Rate = k•[ H2O2]
Since this rate equation is consistent with the experimentally derived equation, the mechanism is considered to be a valid, proposed mechanism.
Catalysts and Reaction Mechanisms
The
decomposition of hydrogen peroxide is abnormally slow. The use of a catalyst can increase the rate of reaction. Catalysts work by changing the mechanism to a lower-energy pathway between reactants and products. Potassium iodide is a commonly used catalyst for the decomposition of H
2O
2. The iodide ion actively participates in the mechanism; the potassium ion is a spectator ion.
The iodide-catalyzed reaction is thought to proceed by the following two-step mechanism:
Step 1: H
2O
2 + I
- → H
2O + IO
-
Step 2: H
2O
2 + IO
- → H
2O + O
2 + I
-
The two steps of this mechanism sum to the following equation:
H2O2 + I- + H2O2 + IO- → H2O + IO- + H2O + O2 + I-
The I
- and IO
- formulae can be cancelled from each side of the equation and like terms can be grouped together with a coefficient to produce the overall balanced equation:
2 H2O2 → 2 H2O + O2
This mechanism has a catalyst (I
-) and an intermediate (IO
-). We previously stated that an intermediate is a product in an early step of the mechanism and a reactant in a later step. On the other hand, a catalyst is a reactant in an early step and a product in a later step. For instance, the catalyst I
- appears as a reactant in step 1 and a product in step 2. The fact that a catalyst is reacted in an early step but produced in a later step is consistent with its definition:
A catalyst is a substance that increases the rate of a chemical reaction without being used up.
While catalysts are never reacted away or consumed, their usefulness diminishes over time due to a phenomenon known as
poisoning. This is particularly true of a heterogeneous catalyst. A heterogeneous catalyst is often a solid catalyst that provides a surface upon which a reaction occurs. A reactant will adsorb or attach to the surface, react on the surface, and then the product is released back to the surroundings. Over time, such catalysts can lose their effectiveness as contaminants (the “poisons”) occupy the sites that reactants normally use. By reducing the number of sites available to catalyze a reaction, the
poison renders the catalyst less useful over the course of time.
Why are Mechanisms Important?
Reaction mechanisms have importance to chemist because they deepen their knowledge of how a reaction occurs. While a balanced chemical equation informs chemists of the stoichiometry of a reaction, the mechanism provides them with a knowledge of the mechanics of a reaction. Such knowledge will help chemists design catalysts, identifying alternative reactants, improving a percent yield, or develop alternative reactions for accomplishing similar purposes at a lower costs. In the end, mechanisms are often the channel through which chemistry can lead to better living.
Before You Leave
- The Check Your Understanding section below include questions with answers and explanations. It provides a great chance to self-assess your understanding.
- Download our Study Card on Reaction Mechanisms. Save it to a safe location and use it as a review tool. (Coming Soon.)
Check Your Understanding
Use the following questions to assess your understanding. Tap the Check Answer buttons when ready.
1. Explain what a reaction mechanism is and how it is different than a balanced chemical equation.
2. Identify the following statements regarding reaction mechanisms as being
TRUE or
FALSE. If
FALSE, correct the statement or explain what is wrong with the statement.
- Reaction mechanisms consists of a variety of reaction steps known as elementary steps.
- The rate of a chemical reaction is determined by the fastest step of the reaction mechanism.
- Intermediates can be either reactants or products in the balanced chemical reaction.
- The number of particles involved in an elementary step is known as the reaction order.
- In a reaction mechanism, a catalyst shows up as a reactant in one of the earlier steps and as a product in a later step.
- By careful experimentation, a chemist can prove what the reaction mechanism is for a given reaction.
- The elementary steps of a reaction mechanism do not need to be balanced.
3. The following reactions represent elementary steps in a variety of mechanisms. For each, write a rate equation based on the elementary step.
a. H
2O
2 + O
→ H
2O + O
2
b. O + NO
2 → NO + O
2
c. O + O
3 → 2 O
2
d. 2 NO
2 → NO
3 + NO
e. Cl + O
3 → ClO + O
2
4. Kent Gettit wrote a reaction mechanism for the reaction:
2 NO2 → 2 NO + O2
His mechanism is a simple two-step mechanism:
Step 1: NO2 + NO2 → NO3 + NO (Slow)
Step 2: NO3 + NO2 → NO + O2 + NO (Fast)
Analyze the mechanism and indicate what is wrong with it.
5. Consider the following two step-reaction mechanism:
Step 1: NO + O2 → NO3 (Slow)
Step 2: NO3 + NO → 2 NO2 (Fast)
a. Identify the balanced chemical equation.
b. Identify the intermediate (if there is one).
c. Identify the catalyst (if there is one).
d. What is the molecularity of the rate determining step?
e. What rate equation would be consistent with this mechanism?
6. Consider the following four step-reaction mechanism:
Step 1: O2 → 2 O
Step 2: O3 + Cl → ClO + O2
Step 3: ClO + O → Cl + O2
Step 4: O3 + O → 2 O2
a. Identify the balanced chemical equation.
b. Identify any intermediates (if there is one).
c. Identify any catalysts (if there is one).
7. Consider the following two step-reaction mechanism:
Step 1: NO2 + NO2 → NO3 + NO (Slow)
Step 2: NO3 + CO → NO2 + CO2 (Fast)
a. Identify the balanced chemical equation.
b. Identify the intermediate (if there is one).
c. Explain why NO
2 is not a catalyst.
d. What is the molecularity of the rate determining step?
e. What rate equation would be consistent with this mechanism?