Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 2: Properties of Matter
Part b: Physical and Chemical Properties
Part 2a: Physical and Chemical Properties
Part 2b: Physical and Chemical Changes
 Investigating Change
Investigating Change
Scientists spend a good deal of time and energy investigating how and why systems change and remain stable. In this part of Lesson 2, we will focus on change. Stuff doesn’t always remain the same; it often experiencing change. We will describe two types of changes – physical change and chemical change.
Physical Changes
 A physical change involves a change in the form or shape or state of a substance without any change in the identity or chemical composition of the substance. When a substance undergoes a physical change, it is the same substance after the change as before the change. An ice cube is water in the solid state. If an ice cube melts, it turns to liquid water. Since there is no change in substance, melting would be considered a physical change.
A physical change involves a change in the form or shape or state of a substance without any change in the identity or chemical composition of the substance. When a substance undergoes a physical change, it is the same substance after the change as before the change. An ice cube is water in the solid state. If an ice cube melts, it turns to liquid water. Since there is no change in substance, melting would be considered a physical change.
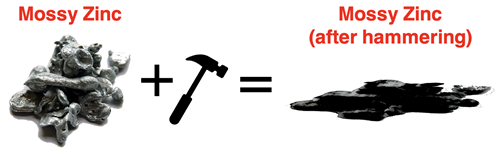
Chemical supply houses often sell the element zinc as mossy zinc. Mossy zinc is fabricated by pouring melted zinc into water so that is solidifies in a fibrous clump with a high surface area (shown below). Suppose you acquire a sample of mossy zinc. You find a hammer and give the mossy zinc a blow. Being a malleable substance, the zinc compresses into a flattened pancake. After hammering, the material is still zinc. This is a simply a change in form or shape. It is a physical change.
Copper can often be purchased as a long coil of wire. Suppose you acquire a sample of coiled copper wire. You find a wire cutter and splice it at various locations. After splicing, you have several short copper wires. The material is still copper. This is a change in form or shape. It is a physical change.
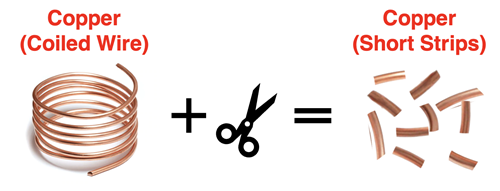
The changes discussed above are all physical changes. There are countless other examples of physical changes that could be discussed. The characteristic feature of any physical change is that the identity of the substance undergoing the change is the same before and after the change. If the change that is being described refers to the formation of a new substance, then that description is not of a physical change. It is a chemical change; chemical changes involve the formation of new substances.
Changes of State
The three states of matter – solid, liquid, and gas – were discussed in Lesson 1 of this chapter of the Chemistry Tutorial. A particle-level description was given for each state. In the solid state, there are strong attractive forces holding particles together as they wiggle about a fixed position. To change a solid to a liquid, those strong forces must be overcome so as to free particles from their fixed position. Increasing the temperature causes particles to vibrate more wildly in their fixed location. At a certain temperature, those vibrations become wild enough to free the particles from their fixed position so that it can move freely in the liquid state. This transition from solid state to liquid state is referred to as melting. Melting is a physical change.
 The liquid state is characterized by particles that are in constant motion. While there are attractive forces between particles, they aren’t strong enough to hold them in a fixed location. Those forces are however strong enough to keep particles from moving about the entire container. Particles in the liquid state never leave the herd to fill the entire volume of the container. As a liquid’s temperature continues to increase, the particles move faster and faster. Some particles at the liquid’s surface have enough energy to overcome the attractive forces and escape the herd and turn to vapor. The liquid is said to have a vapor pressure that depends upon temperature. With continued heating, the vapor pressure of the liquid eventually reaches atmospheric pressure. At this temperature, all particles within the liquid can overcome the attractive forces and the liquid undergoes boiling. With virtually no attractive forces between particles, the gas particles can now expand to fill the container. Like melting, boiling is a physical change.
The liquid state is characterized by particles that are in constant motion. While there are attractive forces between particles, they aren’t strong enough to hold them in a fixed location. Those forces are however strong enough to keep particles from moving about the entire container. Particles in the liquid state never leave the herd to fill the entire volume of the container. As a liquid’s temperature continues to increase, the particles move faster and faster. Some particles at the liquid’s surface have enough energy to overcome the attractive forces and escape the herd and turn to vapor. The liquid is said to have a vapor pressure that depends upon temperature. With continued heating, the vapor pressure of the liquid eventually reaches atmospheric pressure. At this temperature, all particles within the liquid can overcome the attractive forces and the liquid undergoes boiling. With virtually no attractive forces between particles, the gas particles can now expand to fill the container. Like melting, boiling is a physical change.
All changes of state – melting, freezing, boiling, condensation, sublimation, and deposition – are physical changes. If you melt lead, you still have lead atoms. If you boil water, you still have water molecules. Changes of state do not involve a change of the substance to a different substance and that’s why we classify them as physical changes.
Image Credit: Boiling Water
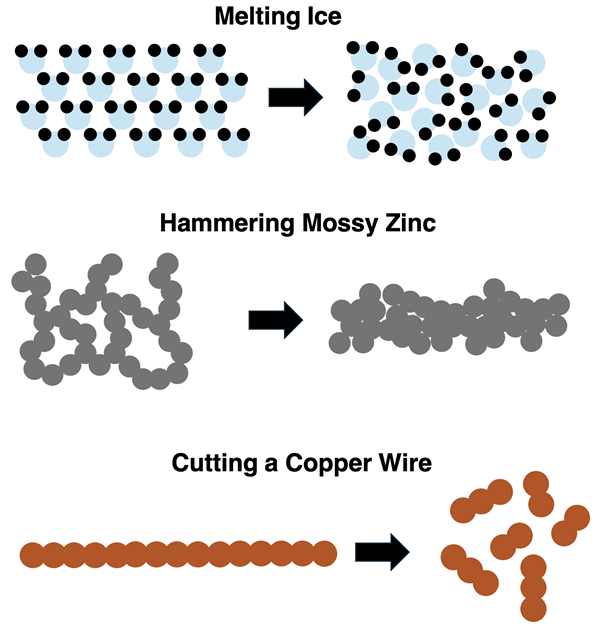
Particle Diagrams for Physical Changes
Particle diagrams are commonly used to represent matter and the changes that it undergoes. The diagrams below represent various physical changes. Note how the substance does not change its identity. It is the same substance before and after the change.
Chemical Changes
Chemical changes involve a chemical reaction. The bonds that hold atoms together in molecules are broken (where applicable) and new bonds between atoms are formed. The reactant chemicals are changed to product chemicals. The reactants are no longer present. The atoms of the reactants have rearranged and the products now exist.

 Chemical changes can occur in seconds or over the course of years. Regardless of how long it takes, the fact that the previously existing reactants are gone and newly formed products now exist means that there will be observable changes. The products may be of a different state as the reactants. The products may have a different color than the reactants, the products may have an odor that the reactants didn’t have. The products may have a different energy level than the reactants. These differences between reactants and products are the reason that chemical reactions leave a trail of evidence – evidence that a reaction has occurred. One or more of the following signs of a chemical reaction are often observed.
Chemical changes can occur in seconds or over the course of years. Regardless of how long it takes, the fact that the previously existing reactants are gone and newly formed products now exist means that there will be observable changes. The products may be of a different state as the reactants. The products may have a different color than the reactants, the products may have an odor that the reactants didn’t have. The products may have a different energy level than the reactants. These differences between reactants and products are the reason that chemical reactions leave a trail of evidence – evidence that a reaction has occurred. One or more of the following signs of a chemical reaction are often observed.
- There is a change in color.
- A solid (precipitate) is formed.
- Gas bubbles are formed.
- An odor is detected.
- The temperature of the surroundings increase or decrease.
- Sound (fizzing, a bang) or light is produced.
Chemical changes have a variety of names. Burning, oxidation, rusting, digesting, synthesis, decomposition, electrolysis, and rotting are a few of the many names given to describe a chemical change. Regardless of the name we use to describe the change, every chemical change involves a change in substance. Reactants turn to products.
Particle Diagrams for Chemical Changes
Earlier we saw article diagrams for physical changes. Now we will look at particle diagrams for chemical changes. Observe how the composition of atoms (the number and type) in the reactants differs from that of the products.
Before You Leave
- Practice. Try our Chemical vs. Physical Properties Concept Builder. It’s great practice!
- The Check Your Understanding section below include questions with answers and explanations. It provides a great chance to self-assess your understanding.
Check Your Understanding
Use the following questions to assess your understanding. Tap the
Check Answer buttons when ready.
1. Consider the following descriptions of change. Identify each as being a chemical or a physical change.
- On a sunny day, chlorophyll in plants absorb sunlight to initiate photosynthesis.
- Two ends of a rope are tied together.
- Wood is burned in a fire pit.
- Solid iodine crystals are heated and undergo sublimation to a gas.
- Lon Skaper throws a large tree trunk into the grinder and it is shredded into small chunks.
- Di Stracted left the milk on the counter before heading to school. When she returned home, it had spoiled.
- The copper disk passed through the rimming machine and a raised rim was added to the disk.
- Mr. H mixed two colorless solutions in a test tube and a yellow solid was formed.
- In advance of preparing a spaghetti meal, Cullen Airry brings liquid water to a boil on an electric stove top.
- It only took a few years before the bottom panel on the Dodge Ram showed significant signs of rusting.
- A bar of molten copper is extruded from the oven in order to produce a long wire.
- One hour after the ingredients were mixed and placed in the oven, the finished cake was removed.
- The burners on the stove are turned on and the natural gas is ignited.
- Indy Gestion consumed a tablespoon of antacid in order to combat his severe indigestion.
- Sand is mixed into the water to form a slurry of sand and water.