Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 2: Properties of Matter
Part a: Physical and Chemical Properties
Part 2a: Physical and Chemical Properties
Part 2b: Physical vs. Chemical Changes
Properties Help Identify Substances
 A property is an attribute, quality, or characteristic associated with an object or class of objects. By observing the properties of an object, you can often identify what the object is. If you see an object on a roadway that has four wheels, a row or two of seats, and a round wheel that can be turned to control the orientation of the wheels, you will conclude the object belongs to the class of objects known as a car. If you see an object with four legs, a furry body, a tail, an occasional bark-like sound, that lives in a house, you will conclude that the object belongs to the class of objects known as a dog. The properties observed of an object help us to classify the object as a car, a dog, a person, a spoon, a pencil, a book, etc. We learn these class-property relationships from an early age and store the information in a remarkable database - our brain.
A property is an attribute, quality, or characteristic associated with an object or class of objects. By observing the properties of an object, you can often identify what the object is. If you see an object on a roadway that has four wheels, a row or two of seats, and a round wheel that can be turned to control the orientation of the wheels, you will conclude the object belongs to the class of objects known as a car. If you see an object with four legs, a furry body, a tail, an occasional bark-like sound, that lives in a house, you will conclude that the object belongs to the class of objects known as a dog. The properties observed of an object help us to classify the object as a car, a dog, a person, a spoon, a pencil, a book, etc. We learn these class-property relationships from an early age and store the information in a remarkable database - our brain.
Chemical substances – elements and compounds - also have properties. The properties that we observe of these substances allow us to classify and identify them. We make observations of their color, their luster, their state (solid, liquid, or gas), their ability to conduct electricity, and whether they are hard or soft, malleable or brittle, rigid or flexible. We make measurements to determine their melting point and boiling point, their density, the degree to which they dissolve in water, etc. We run experiments to observe how they react with other substances. And from all the observations, measurements, and experiments, we create a database of properties for each element and compound. That database is used to help identify the substance that makes up a sample of matter.

Intensive vs. Extensive Properties
An observation is only useful in identifying an unknown substance if it is observable for both small and large samples. If an observed property varies based on the size of the sample, then it has no identifying value. The density, melting point, boiling point, color, and electrical conductivity are examples of properties that are observed to be the same for both small and large samples. We refer to these as intensive properties. Intensive properties do not depend on the amount of substance that is present. Other properties like mass, volume, length, and weight are different for small and large samples. These are called extensive properties. Extensive properties are not useful in identifying substances.
Physical vs. Chemical Properties
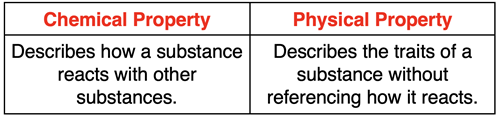
 There are two types of intensive properties that have identifying value. They are called physical properties and chemical properties. A physical property is a trait that is observed or measured without changing the identity of the substance. Put another way, a physical property describes a substance without describing how it reacts with another substance. If a property is not a physical property, then it is a chemical property. A chemical property describes how a substance reacts with another substance.
There are two types of intensive properties that have identifying value. They are called physical properties and chemical properties. A physical property is a trait that is observed or measured without changing the identity of the substance. Put another way, a physical property describes a substance without describing how it reacts with another substance. If a property is not a physical property, then it is a chemical property. A chemical property describes how a substance reacts with another substance.
Physical Properties
Physical properties include observations of appearance such as color and luster (shine). Whether a substance has an odor or is odorless and what type of odor can be detected is a physical property. The state of matter and the temperatures at which a state changes (melting point and boiling point) are physical properties that can be quickly used to identify a substance. Observations of the  electrical and thermal conductivity is an easily observed physical property. A solid object can be tapped with a hammer and observed to either shatter into pieces (brittle), change its shape (malleable), or resist the stress altogether (hard); these are physical properties of materials. Density (to be discussed in full detail in Lesson 4) is another physical property that can be used to identify unknown substances.
electrical and thermal conductivity is an easily observed physical property. A solid object can be tapped with a hammer and observed to either shatter into pieces (brittle), change its shape (malleable), or resist the stress altogether (hard); these are physical properties of materials. Density (to be discussed in full detail in Lesson 4) is another physical property that can be used to identify unknown substances.
The properties discussed above are all physical properties. There are many more that could be mentioned. But the general rule is that a physical property describes the nature of a substance apart from how it reacts with other substances. The moment that a description references how an unknown substance interacts chemically with another substance, it is not a physical property; such a description is a chemical property.
Chemical Properties
Often times one substance will interact or react with another substance. Given unchanging conditions, such reactions are repeatable and predictable and can be considered as an identifying property of the substances involved. New, previously non-existing substances are formed in a chemical reaction; these are referred to as product chemicals or simply products. The products of a reaction are different substances than the original materials (known as reactants). And because they are different substances, they have different physical properties.
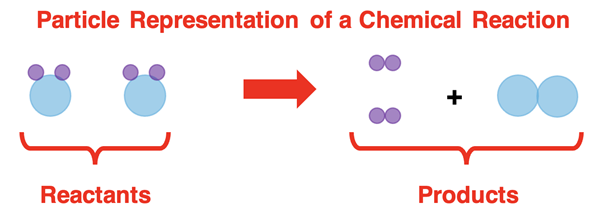
 Chemical reactions can be observed by observing color changes, bubbles forming, odors that were not previously present, a solid precipitating (dropping out or falling), and temperature changes. These macroscopic observables are associated with particle-level events. When reactions occur, the new products may be solids or gases resulting in solid precipitates and the formation of bubbles. The new products may have an odor or a color that is different than the reactants and thus a color change or odor is detected. And finally, reactions involve the breaking of the bonds that hold atoms together in reactant compounds and the forming of new bonds that hold atoms together product compounds. Bond breaking and bond forming require and release energy and this is observed as a change in temperature of the surroundings.
Chemical reactions can be observed by observing color changes, bubbles forming, odors that were not previously present, a solid precipitating (dropping out or falling), and temperature changes. These macroscopic observables are associated with particle-level events. When reactions occur, the new products may be solids or gases resulting in solid precipitates and the formation of bubbles. The new products may have an odor or a color that is different than the reactants and thus a color change or odor is detected. And finally, reactions involve the breaking of the bonds that hold atoms together in reactant compounds and the forming of new bonds that hold atoms together product compounds. Bond breaking and bond forming require and release energy and this is observed as a change in temperature of the surroundings.
 A chemical property of a substance describes how that substance reacts and will often reference many of these observable changes. For the chemist (and the Chemistry student), good detective work allows one to observe color and odor changes, temperature changes, and the presence of bubbles and solid precipitates. When these are observed, one can be relatively certain that the substance is exhibiting one of its chemical properties.
A chemical property of a substance describes how that substance reacts and will often reference many of these observable changes. For the chemist (and the Chemistry student), good detective work allows one to observe color and odor changes, temperature changes, and the presence of bubbles and solid precipitates. When these are observed, one can be relatively certain that the substance is exhibiting one of its chemical properties.
Before You Leave
- Practice. Try our Chemical vs. Physical Properties Concept Builder. It’s great practice!
- The Check Your Understanding section below include questions with answers and explanations. It provides a great chance to self-assess your understanding.
Check Your Understanding
Use the following questions to assess your understanding. Tap the Check Answer buttons when ready.
1. Is it possible for two different substances to have identical chemical and physical properties? Explain why or why not.
2. Several statements were made at the top of the page regarding the element sodium. We have itemized them below. Identify each property as being a chemical or a physical property.
- Soft; easily cuts with knife.
- Solid at room temperature.
- Shiny appearance.
- It quickly dulls to grey when exposed to air.
- Conducts electricity.
- Reacts violently with water! Observed bubbling and flames.
3. Identify the following properties as being either a chemical or a physical property.
- Copper sulfate is a blue solid.
- Chlorine is a yellow-green gas at room temperature.
- Copper metal reacts with nitric acid to produce a green solution and a brown gas.
- Bromine is a reddish-brown liquid at room temperature.
- Bromine boils at approximately 59°C.
- Bubbles are formed when zinc is added to a hydrochloric acid solution.
- Copper is a ductile metal that can be drawn into a long wire without breaking.
- Sulfur is a bright yellow solid.
- Many metals oxidize after prolonged exposure to air.
- Hydrogen sulfide has a rotten egg smell.
- Mixing sodium bicarbonate and acetic acid will result in a profuse amount of bubbles.
- Ammonia gas has a distinctly pungent odor.
- When calcium is added to water, gas and heat is rapidly produced.
- Molten salt is highly conductive.