Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 2: The Quantum Mechanical Model
Part c: Energy Levels
Part 2a: Schrodinger's Wave Mechanical Model
Part 2b: Orbitals
Part 2c: Energy Levels
Part 2d: Quantum Numbers
Principal Energy Levels and Sublevels
 As discussed earlier in this lesson, the Quantum Mechanical Model of the atom proposes that electrons are located in regions of space known as orbitals. Orbitals have precisely known energy (relatively speaking). The principal quantum number (n) determines the principal energy of the orbitals. There are four orbital types of importance – s orbitals, p orbitals, d orbitals, and f orbitals. Each orbital type is considered an energy sublevel within the principal energy level. The first principal energy level has one orbital type – s orbitals. The second principal energy level has two orbital types – s and p orbitals. The s orbital has a lower energy sublevel than the p orbital. The third principal energy level has three orbital types – s, p, and d orbitals. The energy sublevel of the s orbital is lower than the p orbital which is lower than the d orbital. The energy sublevels for the first four energy levels, ordered by energy, are shown below.
As discussed earlier in this lesson, the Quantum Mechanical Model of the atom proposes that electrons are located in regions of space known as orbitals. Orbitals have precisely known energy (relatively speaking). The principal quantum number (n) determines the principal energy of the orbitals. There are four orbital types of importance – s orbitals, p orbitals, d orbitals, and f orbitals. Each orbital type is considered an energy sublevel within the principal energy level. The first principal energy level has one orbital type – s orbitals. The second principal energy level has two orbital types – s and p orbitals. The s orbital has a lower energy sublevel than the p orbital. The third principal energy level has three orbital types – s, p, and d orbitals. The energy sublevel of the s orbital is lower than the p orbital which is lower than the d orbital. The energy sublevels for the first four energy levels, ordered by energy, are shown below.
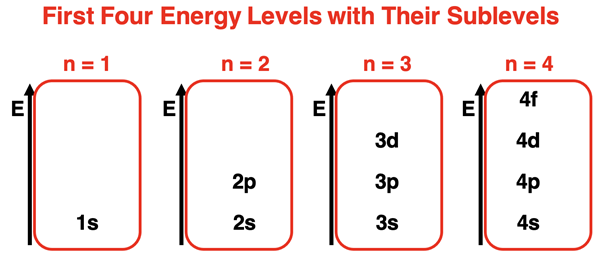
Order of Orbital Energies
Plotting the energy sublevels for the various principal energy levels on the same energy chart yields some surprising results. There is some overlap of energy between consecutive principal energy levels. The overlap is noticeable for any principal energy level containing d orbitals. The s orbital of the next highest energy level is lower in energy than the d orbitals of the previous energy level. As an example, the 4s orbital has lower energy than the 3d orbitals. And the 5s orbital has a lower energy than the 4d orbitals. The pattern continues for other energy levels like those containing the 5d and the 6d orbitals.
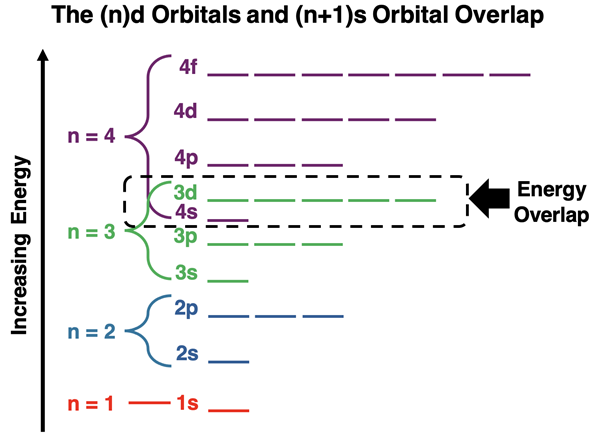
A similar surprise is observed for those energy levels containing f orbitals. The 6s and 5p orbitals are lower in energy than the 4f orbitals. And the 7s and 6p orbitals are lower in energy than the 5f orbitals.
These surprises in the ordering of energy of orbitals can become quite confusing. Predicting which orbitals are lower in energy can get become memory intensive. Fortunately, there are a couple of tricks for remembering the order. The first is presented in the animation below. (The second trick will be presented in Lesson 3.) Begin by listing the energy sublevels for each of the principal energy levels. Then draw diagonals through the listing as shown. The energy ordering is based on which orbitals are reached first by the set of diagonal lines. Study the animation carefully and see if you can repeat it yourself.

The final result is …
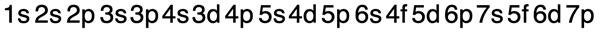
Filling of Orbitals with Electrons
Electrons are located in orbitals. A single orbital can hold a maximum of two electrons. We will now consider the order in which electrons will fill the orbitals for an atom in the ground state. The number of electrons in a neutral atom will equal the atomic number of the element. We will need to give attention to three principles or rules in order to determine the order by which electrons are placed in the orbitals.
- Aufbau Principle: electrons first fill the lowest energy orbitals before beginning to fill orbitals with the next highest energy.
- Hund’s Rule: For sublevels with more than one orbital (p, d, and f), each orbital gets filled with one electron of the same spin direction before pairing up inside of the same orbital.
- Pauli Exclusion Principle: When two electrons are in the same orbital, they will have opposite spin direction.
We will start with some simple cases, applying the above principles to demonstrate the order by which electrons enter the orbitals. The diagrams below are called
orbital box diagrams. Each box represents an orbital. They are ordered in order of energy. To save space, they are organized horizontally. Each orbital is labeled. The arrows represent electrons. Arrows pointing in the same direction have the same spin direction; arrows pointing in opposite directions have opposite spin directions.
Let’s start with the Lithium atom. Lithium has an atomic number of 3. There are three electrons in the neutral atom. The first two electrons fill the lowest energy 1s orbital (Aufbau Principle). The third electron enters the next highest energy level – the 2s orbital. The orbital box diagram is shown below.
Now consider the carbon atom with six electrons. The lowest energy s orbitals (1s and 2s) will hold the first four electrons. The fifth electron will go into a 2p orbital. According to Hund’s Rule, the sixth electron will enter a different 2p orbital and have the same spin direction as the fifth electron. The orbital box diagram is shown below. There are two
unpaired electrons in the 2p orbitals.
The oxygen atom has 8 electrons. The first four electrons will enter the 1s and the 2s orbitals. According to Hund’s Rule, the 5
th, 6
th, and 7
th electron will enter different 2p orbitals and have the same spin direction. After seven electrons, the 2p orbitals are all half-filled. So, the 8
th electron pairs up in a 2p orbital with another electron. According to the Pauli Exclusion Principle, the 8
th electron will spin in an opposite direction as the other p orbital electrons. The arrow is drawn in the opposite direction to indicate a different spin direction. There are two
unpaired electrons in the 2p orbitals.
The magnesium atom has 12 electrons. The first 10 electrons will fill the lowest energy orbitals. In order, the 1s, the 2s, and the 2p orbitals are filled. The 11
th electron enters a 3s orbital. The 12
th electron will also enter a 3s orbital and have the opposite spin direction as the 11
th electron. There are no
unpaired electrons; all electrons are paired up.
The first 12 of the 17 electrons in the chlorine atom will fill (in order) the 1s orbital, the 2s orbital, the 2p orbitals, and the 3s orbital. In accordance with Hund’s Rule, the 13
th, 14
th, and 15
th electron will enter different 3p orbitals. This will half-fill the 3p orbitals. The 16
th and 17
th electron will enter two of these 3p orbitals and pair up with the electrons that are already there. This leaves one
unpaired electron in the 3p orbitals.
The first 18 electrons of potassium’s 19 electrons will enter (in order) the 1s orbital, the 2s orbital, the three 2p orbitals, the 3s orbital, and the three 3p orbitals. Consistent with the Aufbau Principle, the 19
th electron will enter the 4s orbital. The 4s orbital is lower in energy than the 3d orbitals.
Vanadium has 23 electrons. The first 18 electrons will enter (in order) the 1s orbital, the 2s orbital, the three 2p orbitals, the 3s orbital, and the three 3p orbitals. The 19
th and 20
th electrons will enter the 4s orbital. Finally, the 3d orbitals will begin to fill with the 21
st electron. Three of the 3d orbitals will be half-filled with one electron for a total of 23 electrons. There are three
unpaired electrons present in the atom.
Let’s consider a cobalt atom as our final example. It has 27 total electrons. The first 20 electrons will fill in the same way as they did for vanadium. Electrons 21-25 will enter the five different 3d orbitals; none of them will pair up. But the 26
th and 27
th electron will enter an already half-filled orbital to fill it to capacity. There are three
unpaired electrons present in the 3d orbitals and two pairs of paired electrons.
Coming Up
We will revisit these ideas in
Lesson 3 as we learn how to write electron configurations and abbreviated electron configurations. But don’t navigate there too fast. Some Chemistry students are in a class that puts a strong emphasis on quantum numbers. So far, we have only included the bare minimum about quantum numbers. For those who need more, visit
the last page of Lesson 2.
Before You Leave
- Download our Study Card on Orbital Energy Levels. Save it to a safe location and use it as a review tool.
- The Check Your Understanding section below include questions with answers and explanations. It provides a great chance to self-assess your understanding.
Check Your Understanding
Use the following questions to assess your understanding. Tap the Check Answer buttons when ready.
1. For the following pairs of energy sublevels, identify the one that is lowest in energy.
- 2s versus 3s
- 3p versus 3d
- 3p versus 4s
- 3d versus 4s
- 4d versus 4f
- 4f versus 5s
- 4f versus 6s
- 4f versus 5p
- 4f versus 6p
- 4f versus 5d
2. Identify the following statements as being
TRUE or
FALSE.
- A 3s orbital can hold up to 2 electrons. A single 3p orbital can hold up to 6 electrons.
- Two electrons can occupy the same orbital as long as they are spinning in the same direction.
- The orbitals of a principal energy level must be completed filled with electrons before electrons enter the orbitals of the next highest principal energy level.
- The 13th electron of an atom will enter a p orbital. The 14th electron will also enter a p orbital but not the same one as the 13th electron.
- An unpaired electron is an electron that is half-filling an orbital.
- The element arsenic has three unpaired electrons in its 4p orbitals.
3. Identify all the elements in the third period of the Periodic Table have unpaired electrons.
4. Identify all the transition metals in the fourth period of the Periodic Table that have at least one pair of electrons in a d orbital.
5. Identify the transition metal in the fourth period of the Periodic Table that has the greatest number of unpaired electrons.
6. Identify all the elements in the third period of the Periodic Table that have exactly two unpaired electrons in orbitals of the third principal energy level.
7. How many total electron pairs can be found in all the orbitals of sulfur?