Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 1: Intermolecular Forces
Part a: What are Intermolecular Forces?
Part a: What are Intermolecular Forces?
Part b:
Types of Intermolecular Forces
Condensed States
Chapter 10 of our Chemistry Tutorial discussed the nature and behavior of a gas. Particles of a sample of gas are spaced considerably further apart than the particles in a liquid or solid sample. Furthermore, the amount of interaction between particles of an ideal gas have a negligible effect upon the behavior of the gas. Here in Chapter 11, we will be studying a different animal - solids and liquids.

Solids and liquids are considered condensed states of matter. What does it mean to be condensed? When any generic item is condensed, it is made more dense. The volume or space it occupies is reduced without any loss of stuff. In the case of solids and liquids, this means that particles are closer together. This close proximity of particles is accompanied by interparticle forces. The particles attract one another with significant-sized forces and affect the properties of the substance.
Have You Ever Seen a Gas?
 Think on this one: have you ever seen a gas? That is, can you look at a sample of gas and visually detect the stuff in the sample? A sample of air is not visible to the human eye. The air particles are spread too far apart to effectively absorb or reflect visible light to our eye. As such, there is no way to see a sample of air at normal pressure and temperature. A sample of water vapor is also not visible for the same reasons. Wait, you might be thinking, “I’ve seen droplets of water vapor coming out of a tea kettle or a pot on the stove.” It’s always good to be thinking like that, but the fact is that you’ve seen condensed water vapor. That is, what you have seen is water vapor that has cooled down and turned to liquid water droplets that are large enough to reflect light to our eyes. You have seen water in a condensed state.
Think on this one: have you ever seen a gas? That is, can you look at a sample of gas and visually detect the stuff in the sample? A sample of air is not visible to the human eye. The air particles are spread too far apart to effectively absorb or reflect visible light to our eye. As such, there is no way to see a sample of air at normal pressure and temperature. A sample of water vapor is also not visible for the same reasons. Wait, you might be thinking, “I’ve seen droplets of water vapor coming out of a tea kettle or a pot on the stove.” It’s always good to be thinking like that, but the fact is that you’ve seen condensed water vapor. That is, what you have seen is water vapor that has cooled down and turned to liquid water droplets that are large enough to reflect light to our eyes. You have seen water in a condensed state.
Now to be fair, you probably have seen a sample of gas. For instance, you might have seen a sample of greenish chlorine gas or purple iodine gas or brown nitrogen dioxide gas. But most gases that we typically look at are not visible until they condense and form droplets of liquid matter or mix with particulate matter (such as smog or smoke). Liquids and solids - the condensed states - are visible. You can count on that. Their visibility is an indicator of the close proximity of particles that have attracted each other and formed a visible mass.
 Can You Touch a Gas?
Can You Touch a Gas?
We are all generally enamored by the idea of skydiving (as long as we aren’t the ones doing it). Watching a person jump out of the plane at 10000 feet and go spread eagle through the air is fascinating. There’s nothing painful about spread-eagle-ing through air. Particles are spread apart and disconnected. The air particles just move out of the way. But what happens when you try to skydive through water? Say you jump off the 15-foot-tall high dive and do spread eagle towards the water. The fun ends when you reach the water. The water in the pool is in the condensed state. Particles are close together. Particles attract each other with significant interparticle forces. The water particles can’t move out of the way fast enough and your body tells the story. And matters become even worse with solid state materials. Don’t do this at home … or anywhere else.
The fact is that it is really difficult to touch a gas. You can move your hand through the air and the particles are just going to move out of the way. You can put your head do wn and try to run into the air and the particles are just going to move out of the way. You can skydive through a gas. You can run through a gas. And you can drive your car through a gas. The amount of push-back or touching that goes on depends on the speed and cross-sectional area of the object. (And that’s a topic for Physics.)
wn and try to run into the air and the particles are just going to move out of the way. You can skydive through a gas. You can run through a gas. And you can drive your car through a gas. The amount of push-back or touching that goes on depends on the speed and cross-sectional area of the object. (And that’s a topic for Physics.)
Here in Chemistry, we are comparing touching a gas with touching a liquid with touching a solid. And there’s a big difference. Consider this thought experiment. Make a closed fist. Swing it through the air in the room. Then swing it through the water in a deep pool. Then remember this is a thought experiment. Finally, as part of the thought experiment, swing your fist through the solid ground. What did you observe? You likely observed in the three trials that the particles kept getting closer together and the forces between the particles kept getting stronger.
Intermolecular Forces for Molecular Compounds
 It should be evident from the above discussion that solids and liquids are quite different than gases. The condensed states owe much of these differences to intermolecular forces. The topic is a big enough deal in Chemistry that we devote a chapter to it … and we will return to the concept many times in subsequent chapters. So, what are intermolecular forces?
It should be evident from the above discussion that solids and liquids are quite different than gases. The condensed states owe much of these differences to intermolecular forces. The topic is a big enough deal in Chemistry that we devote a chapter to it … and we will return to the concept many times in subsequent chapters. So, what are intermolecular forces?
Intermolecular forces are the forces of attraction (and repulsion) that exist between molecules in molecular compounds. They are the forces that pull molecules of liquid water towards one another as you try to drive your fist through the water of the pool. They are the forces that hold individual molecules of solid water together to form the unique shape of an ice crystal. Intermolecular forces are electrical in nature. That is, they are the result of opposite charges attracting and like charges repelling. We will dig deeper into the concept of charges and electrical attractions on the next page of Lesson 1 when we discuss the three types of intermolecular forces.
 Intermolecular forces should not be confused with intramolecular forces. Inter- is not intra-. The inter- of intermolecular forces describes the forces that exist between or among different molecules. Intermolecular forces hold one molecule to another molecule. Just as interscholastic athletics involve athletic events between two different schools, intermolecular forces involve forces between two different molecules. On the other hand, the intra- of intramolecular forces describes the forces within or inside of a molecule. Intramolecular forces are the bonds that hold atoms together within a molecule. Just as intramural athletics are athletic events between two teams within the same school, intramolecular forces are the forces that hold two atoms within the same molecule together.
Intermolecular forces should not be confused with intramolecular forces. Inter- is not intra-. The inter- of intermolecular forces describes the forces that exist between or among different molecules. Intermolecular forces hold one molecule to another molecule. Just as interscholastic athletics involve athletic events between two different schools, intermolecular forces involve forces between two different molecules. On the other hand, the intra- of intramolecular forces describes the forces within or inside of a molecule. Intramolecular forces are the bonds that hold atoms together within a molecule. Just as intramural athletics are athletic events between two teams within the same school, intramolecular forces are the forces that hold two atoms within the same molecule together.
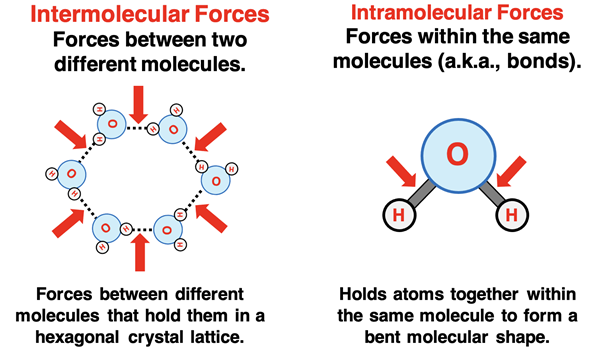
Comparing Intermolecular Forces with Intramolecular Forces
Let’s consider for a moment the act of vaporizing water molecules. This is the process of turning liquid water into gaseous water. To do so, the intermolecular forces that hold liquid water molecules together must be overcome. Energy is required to pull the molecules apart. And careful measurement through the years has informed us that roughly 41 units of energy are required for every one mole of water molecules. Technically, the value is 40.6 kilojoules per every mole of water molecules undergo vaporization. This figure, referred to as the heat of vaporization, provides a measure of the strength of the intermolecular forces. Now let’s contrast this strength with the strength of the intramolecular forces or bonds between water molecules.
A water molecule is composed of two H atoms and one O atom held together by two H-O bonds. The bonds can be broken by the input of energy. The energy is used to overcome the intramolecular forces and to pull the individual atoms apart. The energy to break both bonds in a liquid water molecule is a measurable quantity.

Careful measurement shows that the above process requires 926 units of energy for every one mole of liquid water molecules. Technically, the value is 926 kilojoules for the breaking of both O-H bonds in every mole of water molecules. This figure provides a measure of the strength of the intramolecular forces holding atoms together within a water molecule.
So, what do we learn from this mess of data? In comparing the 926 kJ of energy (per mole) to overcome the intramolecular forces of the two H-O bonds to the 40.6 kJ of energy (per mole) to overcome the intermolecular forces of liquid water molecules, we can conclude that bonds are significantly stronger than intermolecular forces. Chemical changes generally require more energy than physical changes. Decomposing liquid water into its elements by the breaking of the bonds is a much more difficult task than boiling liquid water and changing it into water vapor.
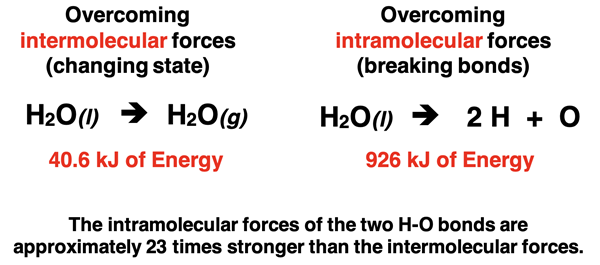
Importance of Intermolecular Forces
The so-called IMFs (our shorthand for intermolecular forces) are amateurs in comparison to the stronger bonds that hold atoms together in molecular compounds. But nonetheless, IMFs are considerably important. As we will see in Lesson 2, they determine many of the properties of liquids. And in Lesson 3, we will learn that they determine the structure and properties of solids. But before we continue forward to our study of liquids and solids, we need to learn more about the three types of intermolecular forces. That’s the topic of the next page of Lesson 1.
Before You Leave
- The Check Your Understanding section below include questions with answers and explanations. It provides a great chance to self-assess your understanding.
Check Your Understanding
Use the following questions to assess your understanding. Tap the Check Answer buttons when ready.
1. Which states of matter are considered the condensed states?
2. Explain why it is difficult to see a gas like air but solids and liquids can be seen.
3. Explain why it is difficult to
touch a gas like air but solids and liquids are easily touched.
4. A bond between atoms in a molecular compound is sometimes referred to as a ______.
a. intermolecular force
b. intramolecular force
5. Describe the difference between intermolecular forces and intramolecular forces.
6. Which type of force tends to be stronger - intermolecular forces or intramolecular forces?