Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 1: Ionic Bonding
Part b: Ions and Ionic Bonds
Part 1a:
Electronegativity and Bond Types
Part 1b: Ions and Ionic Bonds
Part 1c:
Ionic Compounds
Ion Charges Revisited
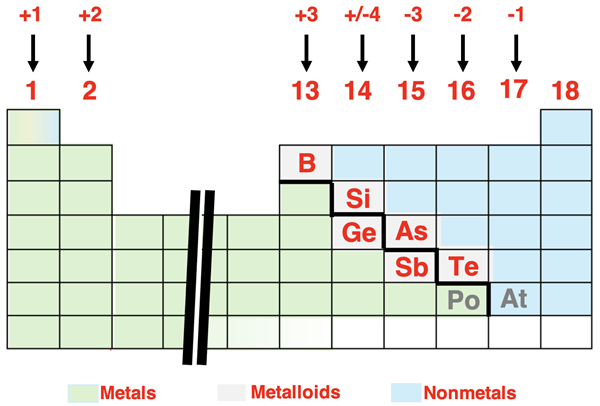
We first learned about ions in Chapter 3 of this Chemistry Tutorial. Specifically, we learned that main group elements on the Periodic Table form ions with predictable charges. Groups 1, 2, 13, and 14 metals form 1+, 2+, 3+, and 4+ ions respectively. Group 15, 16, and 17 nonmetals form 3-, 2-, and 1- ions respectively. The patterns are summarized in the diagram below. There are some notable exceptions to this general pattern. Examples include tin (50Sn) and lead (82Pb), which can form 2+ ions in addition to the predicted 4+ ions.
What we didn’t learn in Chapter 3 was the reason why such patterns exist. The why? questions are the important questions … and the interesting questions in Chemistry. As is usually the case, the why? questions get answered when we look at matter at the particulate level. Get out your magnifying glass … it’s time to zoom in.
 Noble Gas Stability
Noble Gas Stability
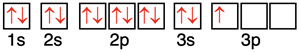
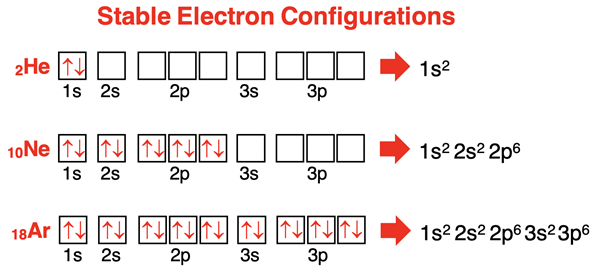
We mentioned on the previous page of Lesson 1 that every atom wants to be like HeNeArKrXeRn. Every atom would like to gain, share, or lose electrons in order to acquire the electron configuration of a noble gas. Noble gas elements each have an s2p6 electron configuration with eight valence shell electrons. (Helium is the one exception; it has an s2 configuration and only two valence shell electrons.) The s and p orbitals of a noble gas are completely full. A full octet of valence shell electrons is a stabilizing feature of any atom and any ion. Noble gases are the most inert (non-reactive) elements that exist. They have little to nothing to gain by forming bonds with other elements. They have little to nothing to gain because they are energetically stable as is.
 Our best explanation for why any two atoms would form a bond is that they are more energetically stable when combined than when they exist as individual atoms. Such stability is acquired when they obtain eight valence shell electrons. This is known as the octet rule. An atom of a main group element will gain, lose, or share electrons in order to acquire an octet of valence shell electrons. For metals in the second period, the nearest noble gas is helium with two valence shell electrons. Such metals (Li and Be) will follow the duet rule.
Our best explanation for why any two atoms would form a bond is that they are more energetically stable when combined than when they exist as individual atoms. Such stability is acquired when they obtain eight valence shell electrons. This is known as the octet rule. An atom of a main group element will gain, lose, or share electrons in order to acquire an octet of valence shell electrons. For metals in the second period, the nearest noble gas is helium with two valence shell electrons. Such metals (Li and Be) will follow the duet rule.
Main Group Elements and Valence Shell Electrons
Both main group metals and nonmetals will form ions in order to achieve these stable octets. We learned in Chapter 5, that the number of valence electrons in a main group element depends on the element’s location on the periodic table. The table below shows the abbreviated electron configuration for all main group elements. The valence shell electrons – s and p electrons in the outermost shell – are highlighted in red. Scan any of the columns and observe that every element in that column has the same number of valence electrons.
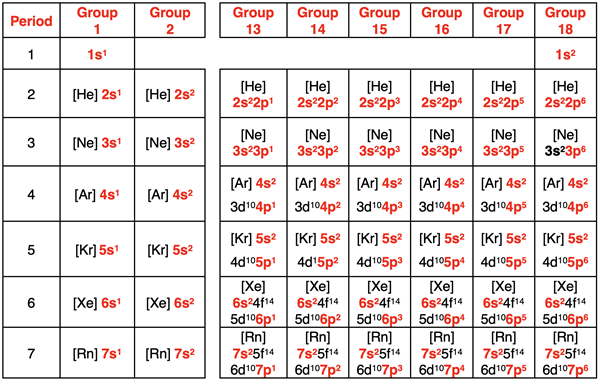
Main group metals in periods 1-4 contain three or less valence shell electrons. In period 5 and higher, there are metals with four or more valence shell electrons. These larger metals are more likely to be multivalent (form ions with different charges) or deviate from the general patterns for the charges on ions. In contrast to metals, most main group nonmetals contain 5 or more valence shell electrons. Carbon contains four valence shell electrons.
Main Group Metals
Now let’s discuss how the octet rule can explain the charges acquired by main group elements when they form ions. Metals lose electrons to form positively charged ions (cations). For metals, losing electrons is the lowest-energy pathway to acquiring stable octets of electrons.
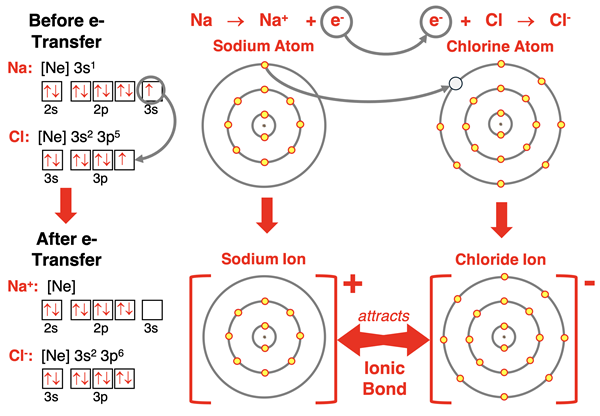
Sodium (and all group 1 elements) has an s1 electron configuration. When it loses the 3s1 electron, its outer shell becomes the n=2 shell which has a set of s and p orbitals completely filled with electrons. Sodium forms a 1+ ion with the electron configuration of neon – the noble gas at the end of the row above it. This same pattern of ion formation is followed by all alkali metals (group 1) elements.
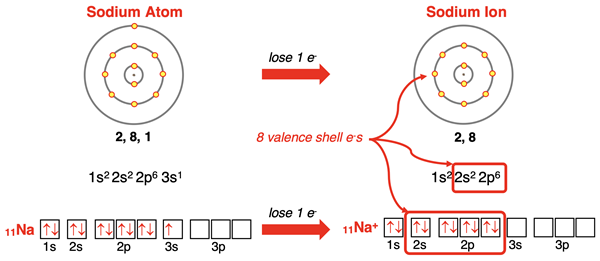
The formation of a 1+ ion by sodium is often represented by the symbolic notation below. This is interpreted as a sodium atom (left side of →) forms a sodium 1+ ion plus one electron.

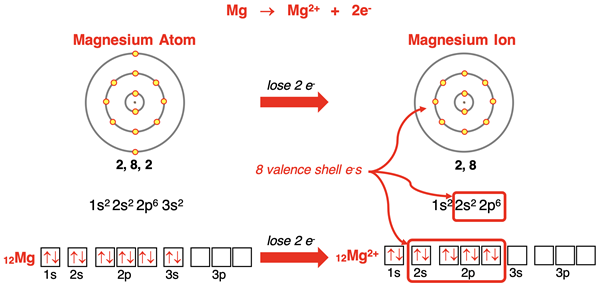
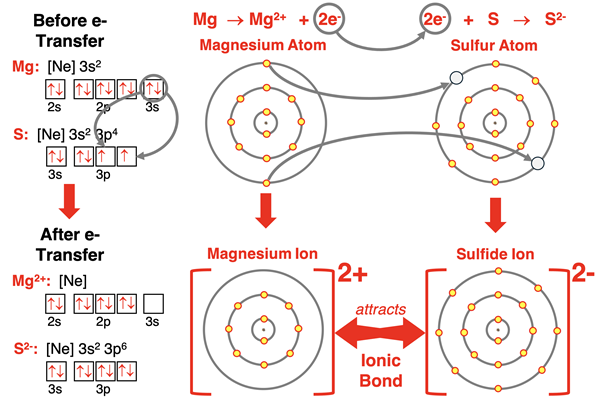
Magnesium (and all group 2 elements) has an s2 electron configuration. When it loses the two electrons in its 3s orbitals, its outer shell becomes the n=2 shell. This shell already has a set of s and p orbitals filled with electrons, giving the ion an octet of electrons. Magnesium forms a 2+ ion with the electron configuration of neon. This same pattern of ion formation is followed by all alkaline earth metal (group 2) elements.
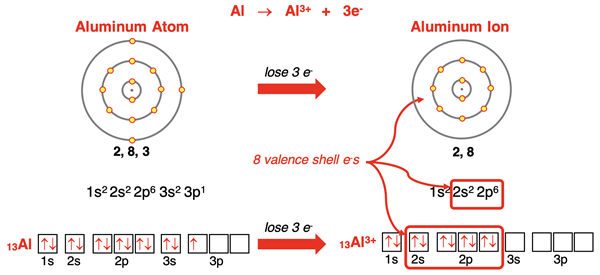
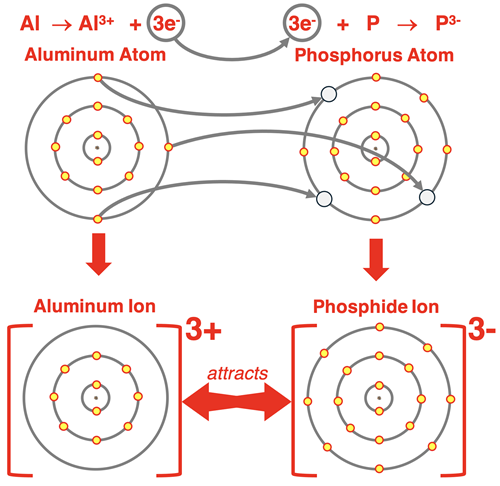
Aluminum has an s2p1 electron configuration. When it loses its three valence shell electrons, its outer shell becomes the n=2 shell. This gives the Al3+ ion an octet of valence shell electrons and the stable electron configuration of neon. This same pattern of ion formation is followed by other group 13 metals - gallium (31Ga), indium (49In), and thallium (81Tl). (Thallium more commonly forms a 1+ ion. The irregularity is often attributed to an effect known as the inert pair effect.)
Main Group Nonmetals
Main group nonmetals are more than halfway towards an octet of electrons. As such, gaining electrons is the lowest-energy pathway to acquiring stable octets of electrons. So, the nonmetals in groups 15-17 gain electrons to form negative ions (anions) and acquire the electron configuration of a noble gas.
Phosphorus (and nitrogen) has an s2p3 electron configuration. With five valence shell electrons, it will need to gain three more electrons in order to acquire an octet of electrons in its outermost shell. After gaining three electrons, the phosphide ion has an s2p6 electron configuration. All s and p orbitals of its valence shell are full. It has argon’s noble gas electron configuration. A nitrogen atom would behave similarly for the same reason and acquire neon’s electron configuration.

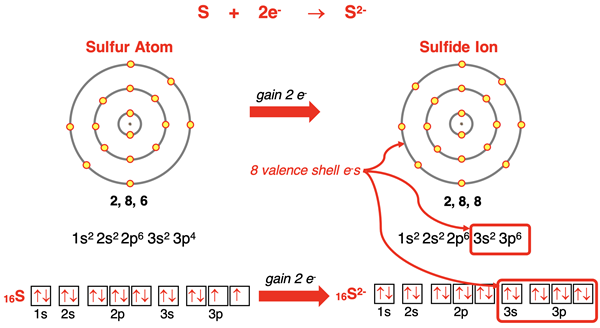
Sulfur (and oxygen and selenium) has an s2p4 electron configuration. It has six valence shell electrons. Sulfur must gain two more electrons to acquire an octet of electrons in its outermost shell. When it gains two electrons to become the S2- ion, it will have an s2p6 electron configuration with completely full s and p orbitals in its outermost electron shell. The sulfide ion has argon’s noble gas electron configuration. Oxygen and selenium atoms would behave similarly for the same reason, acquiring a 2- charge and a noble gas electron configuration.
Chlorine (and the other halogens of group 17) has an s2p5 electron configuration. The chlorine atom is one electron shy of having an octet of electron in its valence shell. By gaining an additional electron, it will have the noble gas configuration of argon with completely full s and p orbitals in its n=3 electron shell. Atoms of fluorine, bromine, and iodine will behave similarly for the same reason.
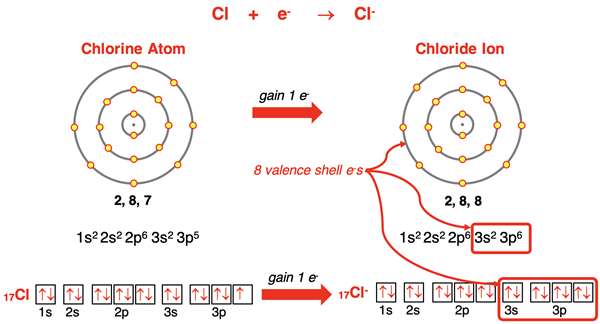
Electron Transfer
The loss of an electron by a metal and the gain of an electron by a nonmetal are often simultaneous events. A better description than loss and gain would be transfer. The metal transfers the electron(s) to the nonmetal. The metal gets some help from the more electronegative nonmetal as it pulls the electron(s) towards itself. The ions that are formed as the result of the transfer are oppositely charged. Their attraction for one another is strong enough to hold the ions together as a single unit. An ionic bond is formed.
The arrangement for the sodium atom and the chloride atom is very straightforward. Sodium has one valence shell electron it would like to lose. Chlorine would like to gain one valence shell electron. So, the 3s1 electron on sodium transfers to chlorine’s half-filled 3p orbital. The positively charged sodium ion and the negatively charged chloride ion are formed, each with a full octet of electrons. The attraction between oppositely charged ions occurs and an ionic bond is formed. This process is shown below with symbols, electron configurations, orbital box diagrams, and electron shell diagrams.
The arrangement for the magnesium atom and the sulfur atom is equally straightforward but there’s twice as many electrons involved. Magnesium has two valence shell electrons it would like to lose. Sulfur would like to gain two valence shell electrons. So, both 3s electrons on magnesium transfer to each of sulfur’s half-filled 3p orbitals. The positively charged magnesium ion and the negatively charged sulfide ion are formed, each with a full octet of electrons. The attraction between oppositely charged ions occurs and an ionic bond is formed. This process is once again shown below with symbols, electron configurations, orbital box diagrams, and electron shell diagrams.
After the first two examples, you can likely figure out the arrangement for an aluminum atom with three valence shell electrons and a phosphorus atom with five valence shell electrons. Three electrons must be transferred from the aluminum atom to the phosphorus atom. The transfer is shown below. An ionic bond is formed.
We have analyzed three rather straightforward electron transfer situations. In each case, the number of electrons lost by the metal was equal to the number of electrons gained by the nonmetal. But what happens if they are not equal? For instance, what happens if magnesium meets up with fluorine? A magnesium atom wants to transfer two electrons, but a fluorine atom only wants to gain one electron. How can that possibly work? Easy. Just double the number of fluorine atoms. Magnesium has enough electrons to lose to make two fluorine atoms quite satisfied.
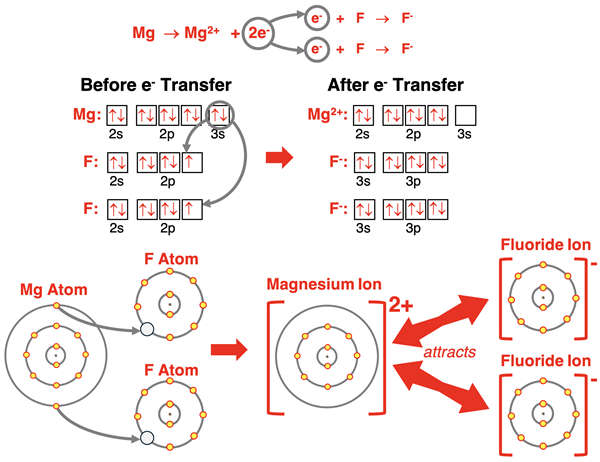
In our final page of Lesson 1, we will take the concept of ionic bonding one step further and discuss ionic compounds and their structure.
Before You Leave
- Practice. Try our Ionic Bonding Concept Builder. It’s great practice!
- Download our Study Card on Ionic Bonding. Save it to a safe location and use it as a review tool.
- The Check Your Understanding section below include questions with answers and explanations. It provides a great chance to self-assess your understanding.
Check Your Understanding
Use the following questions to assess your understanding. Tap the Check Answer buttons when ready.
1. What is meant by the comment “every atom wants to be like HeNeArKrXeRn”?
2. Explain what an octet of electrons is?
3. Complete the following statements:
- An atom with a [Ne]3s2 electron configuration will _______ (gain, lose) _______ (a number) electron(s) to achieve the electron configuration of the element ___________.
- An atom with a [Ne]3s23p5 electron configuration will _______ (gain, lose) _______ (a number) electron(s) to achieve the electron configuration of the element ___________.
- An atom in period 4 with six valence shell electrons will _______ (gain, lose) _______ (a number) electron(s) to achieve the electron configuration of the element ___________.
- An atom in period 3 with three valence shell electrons will _______ (gain, lose) _______ (a number) electron(s) to achieve the electron configuration of the element ___________.
4. Consider the following electron configurations. For each, identify the charge of the ion that the element would form.
- [Kr] 5s2
- [Ar] 4s1
- [He] 3s2 3p3
- [Kr] 5s2 4d10 5p5
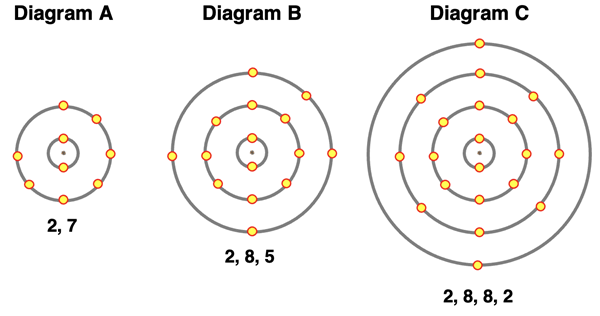
5. Consider the electron shell diagrams below. For each, identify whether the atom would gain or lose electrons, the number of electrons gained or lost, and the resulting charge of the ion.
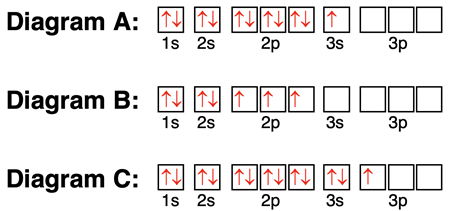
6. Consider the orbital box diagrams below. For each, identify whether the atom would gain or lose electrons, the number of electrons gained or lost, and the resulting charge of the ion.
7. Atoms A and B will be forming an ionic bond. Their electron configurations are shown. Which atom – A or B – loses electrons and which gains electrons. How many electrons are gained or lost? And what are the resulting charges of the ions?

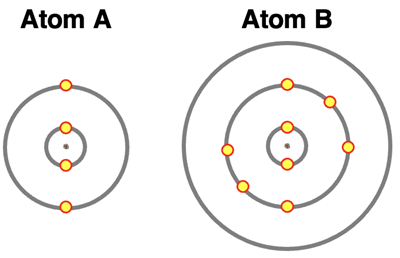
8. Assume that atoms A and B will be forming an ionic bond. Their electron shell diagrams are shown. Which atom – A or B – loses electrons and which gains electrons. How many electrons are gained or lost? And what are the resulting charges of the ions?
9. Consider the orbital box diagram below. How many fluorine atoms would combine with this atom in an ionic compound? Explain your answer.