Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 1: In Search of the Atom
Part a: Democritus to Dalton
Part 1a: Democritus to Dalton
Part 1b: The Inside Story of the Atom
Part 1c: Subatomic Particles
Breaking Down the Atom
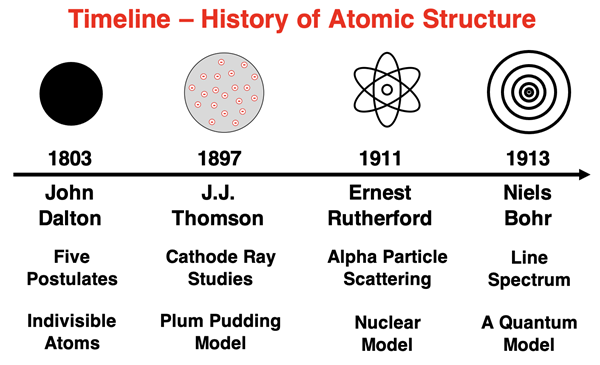
As mentioned earlier in Lesson 1, John Dalton’s atomic theory (1803) presented five postulates regarding atoms and their interaction. The theory promoted an atom-based framework for thinking about elements and their relative mass, compounds and their composition, and chemical reactions. One of the postulates of the theory was that atoms were indivisible. Put another way, there was nothing smaller than an atom; it could not be broken down into even smaller parts. But were atoms really indivisible? Was an atom a miniaturized billiard ball with no internal contents or structure? Or was there something even smaller that was contained within the atom that could be discovered? And if so, how can one discover details about that which is too small to be seen?
The Plum Pudding Model
In the late 19th century, a considerable amount of scientific research centered around the use of cathode ray tubes and the investigation of cathode rays. English physicist J.J. Thomson (1899) researched the rays emitted from the cathode. Using both a magnetic field and an electric field to deflect the path of the rays, Thomson determined that they consisted of a stream of negatively charged particles. These particles were named electrons. J.J. Thomson eventually determined that the mass of an electron was 1/1000-th of the mass of a hydrogen atom.
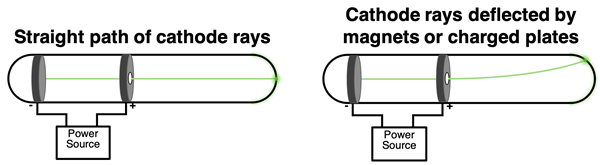
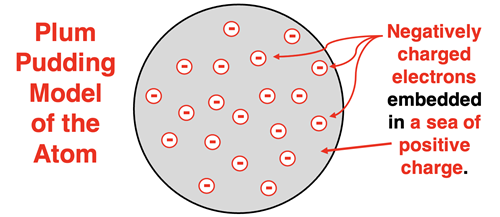
Thomson reasoned that since the electrons were being emitted by the atoms of the metal cathode, then those electrons must be located in those atoms. Thomson developed the first structural model of the atom, proposing that an atom contains negatively charged electrons. These electrons were distributed about the atom. And since an atom is electrically neutral, the charge of the electrons must be balanced by an equal quantity of positive charge. The positive charge was distributed uniformly throughout the atom. So, the Thomson model pictured an atom as consisting of negatively charged electrons embedded in a sea of positive charge. The model later became known as the Plum Pudding Model of the atom. The electrons were like raisins and the sea of positive charge was like the plum of England’s favorite desert.
Alpha Particle Scattering Experiment
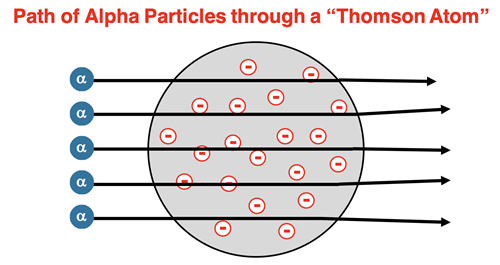
 Nearly a decade after J.J. Thomson’s announcement of the electron, his student Ernest Rutherford was conducting a set of experiments with alpha particles. Rutherford beamed alpha particles at a very thin foil of gold. Alpha particles are very small, positively charged particles. If Thomson’s model of the atom was correct, it would be expected that the stream of alpha particles would pass straight through the gold foil. There would be a variable, but minimal amount of deflection from their original path. The majority of alpha particles did just that. But there were many particles deflected at large angles. Some particles even rebounded backwards at angles that exceeded 90° relative to the original path. Rutherford described his amazement regarding the rebounding particles.
Nearly a decade after J.J. Thomson’s announcement of the electron, his student Ernest Rutherford was conducting a set of experiments with alpha particles. Rutherford beamed alpha particles at a very thin foil of gold. Alpha particles are very small, positively charged particles. If Thomson’s model of the atom was correct, it would be expected that the stream of alpha particles would pass straight through the gold foil. There would be a variable, but minimal amount of deflection from their original path. The majority of alpha particles did just that. But there were many particles deflected at large angles. Some particles even rebounded backwards at angles that exceeded 90° relative to the original path. Rutherford described his amazement regarding the rebounding particles.
It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you.
 Rutherford knew that charged particles interact with one another. So, the positively charged alpha particles would interact with the negatively charged electrons and with the sea of positive charge. But given the high speed of the particles bombarding the gold foil, any significant deflection from the original path could only be caused by a relatively high concentration of charge within the atoms of gold. The Thomson model of the atom had both the negative electrons and the sea of positive charge spread evenly about the spherical volume of the atom. Rutherford knew this distribution could never deflect an alpha particle at angles greater than 90° (or even at angles much greater than 10°).
Rutherford knew that charged particles interact with one another. So, the positively charged alpha particles would interact with the negatively charged electrons and with the sea of positive charge. But given the high speed of the particles bombarding the gold foil, any significant deflection from the original path could only be caused by a relatively high concentration of charge within the atoms of gold. The Thomson model of the atom had both the negative electrons and the sea of positive charge spread evenly about the spherical volume of the atom. Rutherford knew this distribution could never deflect an alpha particle at angles greater than 90° (or even at angles much greater than 10°).
 The Nuclear Model
The Nuclear Model
Rutherford’s revised the atomic model. He proposed that the positive charge was concentrated in a nucleus and not spread evenly about the atom. With a high concentration of positive charge, the electrical repulsions between the nucleus and the alpha particles would be significant enough to deflect alpha particles at greater angles. The closer that the alpha particles came to the nucleus, the more that they felt the force and the greater the deflection. And for head-on paths towards the nucleus, the alpha particle would be rebounded back towards the source.
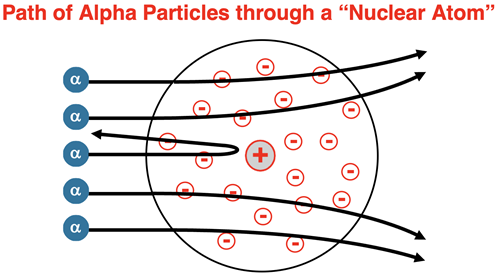
Rutherford proposed that the positive charge was due to the presence of a subatomic particle known as the proton. The proton had roughly the mass of a hydrogen atom, making it 1000 times more massive than an electron. As such, most of the mass of an atom was concentrated in the nucleus. The proton had an amount of positive charge equal to the amount of negative charge on an electron. Atoms of different elements had a different number of protons in their nucleus. The number of protons would be equal to the number of electrons surrounding the nucleus in order to provide an overall neutral atom. The planetary model of the atom is most often attributed to Rutherford and has become an icon of science ever since.
The Electron Problem
 Rutherford’s nuclear model provided an explanation for the alpha particle scattering experiment. But Rutherford was aware of the obvious issues with the model. The issues pertained to those electrons. What exactly were the electrons doing? If they had an opposite charge as the nucleus, then they would be attracted towards the nucleus and the atom would collapse. Rutherford proposed that the electrons were in orbit about the nucleus much like the planets orbit the sun. Rutherford knew that placing the electrons in orbit was not the solution to the problem. It was known that a moving, charged particle would continuously emit electromagnetic radiation. This loss of energy would cause the electron to spiral into the nucleus quite quickly.
Rutherford’s nuclear model provided an explanation for the alpha particle scattering experiment. But Rutherford was aware of the obvious issues with the model. The issues pertained to those electrons. What exactly were the electrons doing? If they had an opposite charge as the nucleus, then they would be attracted towards the nucleus and the atom would collapse. Rutherford proposed that the electrons were in orbit about the nucleus much like the planets orbit the sun. Rutherford knew that placing the electrons in orbit was not the solution to the problem. It was known that a moving, charged particle would continuously emit electromagnetic radiation. This loss of energy would cause the electron to spiral into the nucleus quite quickly.
There was another problem. But before we discuss it, let's lighten up and do a short Physics lesson.
Shedding Light on the Topic
During the early 1900s, a considerable amount of physics research was being devoted to understanding light, matter, and the interaction between light and matter. Light is an electromagnetic wave that has a range or spectrum of wavelengths and frequencies. Higher wavelengths correspond to lower energy radiation. A portion of the electromagnetic spectrum of wavelengths includes light that is visible to the human eye. We creatively refer to this as the visible light spectrum. Visible light, when separated into its varying wavelengths, is perceived as a red, orange, yellow, green, blue, indigo, and violet light.
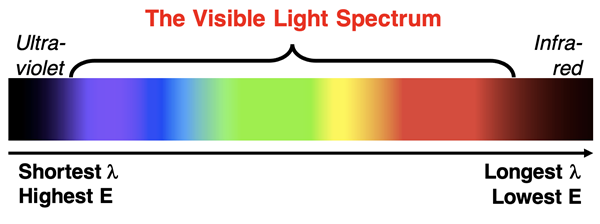
This continuous spectrum of colors can be remembered by the mnemonic ROY G BIV. Each letter of ROY G BIV represents one of the component colors, organized from longest wavelength (Red) to shortest wavelength (Violet). Red light has the lowest energy. Electromagnetic radiation with wavelengths just greater than red fall outside the visible light spectrum are referred to as infrared radiation. Violet light has the highest energy. Electromagnetic radiation with wavelengths just smaller than violet fall are referred to as ultraviolet radiation.
Scientists knew that atoms of gaseous elements absorbed and released electromagnetic energy (light) when heated or exposed to high electric voltages. Such absorptions and emissions occurred for specific wavelengths of light. Some of these wavelengths were within the ROY G BIV spectrum. The result was that each element had its own unique line spectrum. The line spectrum for hydrogen is shown below. Each line indicates wavelengths (or colors) of light that are absorbed or emitted by atoms of that element.
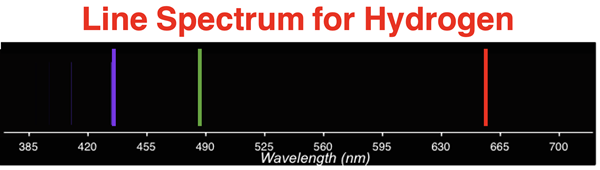
It was believed the absorption and emission events were associated with changes in electron behavior. Rutherford’s atomic model was incapable of explaining the connection between the line spectra of elements and the nature of the electron’s orbits.
Learn more about: Emission Spectrum of the Elements
The Bohr Model
 In an effort to explain the line spectra, Niels Bohr (1913) proposed a quantum model of the atom. Bohr suggested the electron could only orbit the nucleus with certain specific energies. Each energy is associated with an orbital radius. The higher energy levels are associated with larger radius orbits. Because the energies and radii of orbits were fixed values, it was not possible for the electron to spiral from one orbit to another orbit. The electron’s radius could be one value or another value, but not any value in between. The energies were quantized and not continuous.
In an effort to explain the line spectra, Niels Bohr (1913) proposed a quantum model of the atom. Bohr suggested the electron could only orbit the nucleus with certain specific energies. Each energy is associated with an orbital radius. The higher energy levels are associated with larger radius orbits. Because the energies and radii of orbits were fixed values, it was not possible for the electron to spiral from one orbit to another orbit. The electron’s radius could be one value or another value, but not any value in between. The energies were quantized and not continuous.
In the Bohr quantum model, an electron could make a quantum jump from one energy level to another. By absorbing electromagnetic light of a specific radiation, the electron could jump upward to an orbit of a higher energy level. And by emitting (releasing) electromagnetic radiation of a specific energy, the electron could jump downward to an orbit of a lower energy level. The amount of absorbed or emitted energy must equal the difference in energy of the two orbits between which the electron is jumping. If the two orbits between which the electrons are jumping are separated by a large amount of energy, then higher energy EM radiation will be emitted (or absorbed); this might correspond to violet light or even ultraviolet radiation. A lower energy electron transition might correspond to red light or even infrared radiation.
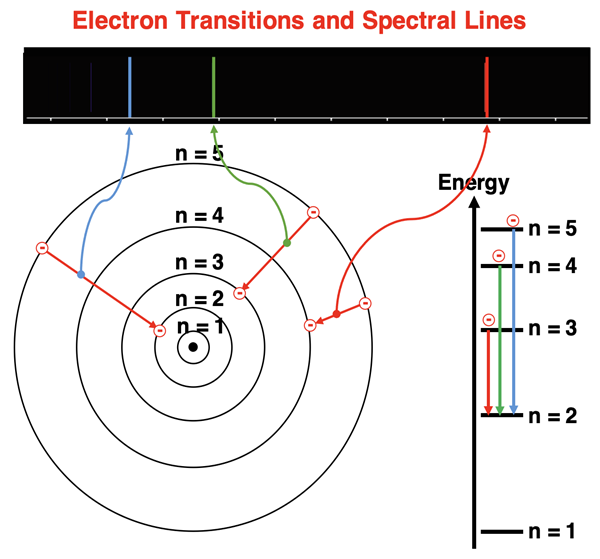
Bohr’s model was very mathematical. It was derived from the mathematics of circular motion and charge interactions. The derived equations could predict the energies of the lines in the spectra of various elements. The predictions worked well for the hydrogen atom (the smallest of all atoms). But the failure to match his predictions to the spectral lines for other elements was an indicator that more work was needed on the theory.
Learn more about: Bohr's Quantized Energy Levels
Moving Closer to the Modern Model of the Atom
John Dalton’s Atomic Theory provided good reason for believing in the existence of atoms. But the Dalton atom was considered indivisible with no internal structure. The findings of Thomson and Rutherford demonstrated that the atom was not indivisible, but was instead composed of smaller parts. The relationship between the parts – their mass, charge, location, and interaction – was slowly becoming discovered during the early decades of the 20th century. Niels Bohr use of quantized energy levels was another step closer to what would eventually become known as the modern model of the atom. We will continue the story in Chapter 5 of this Chemistry Tutorial when we explore the quantum mechanical model.
Before You Leave
- There’s nothing better to help organize the ideas – names, model, experiment, concept – than our Atomic Models Concept Builder.
- Refine your understanding of the Bohr model and try our Line Spectra Concept Builder.
- The Check Your Understanding section below include questions and problems with answers and explanations and solutions. It provides a great chance to self-assess your understanding.
Check Your Understanding
Use the following questions to assess your understanding. Tap the
Check Answer buttons when ready.
1. Consider the following statements. Match each statement to the scientist – Dalton, Thomson, Rutherford, Bohr - first responsible for the idea.
- Electrons orbit the nucleus with quantized energy levels.
- Atoms contain negatively charged particles called electrons.
- Atoms contain a positively charged nucleus.
- Chemical reactions can be explained by the existence of atoms.
- Atoms are not indivisible but contain smaller parts.
- Electrons can move from one orbit to another by absorbing or releasing electromagnetic radiation.
- Atoms are indivisible building blocks from which matter is built.
- Most of the mass of an atom is concentrated in its nucleus.
- Atoms contain positive and negative charge in equal amounts.
2. The energy of electromagnetic radiation released by an electron as it transitions from one energy level to another is equal to _____.
- the lowest energy an electron can have
- the change in energy the electron experiences
- the speed at which it travels once emitted
- the energy the electron had before the radiation release
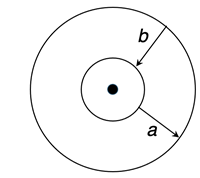
3. Bohr pictured electrons as orbiting the nucleus in circular orbits. Each orbit had its own energy level. Inner orbits have lower energy levels. Outer orbits have high energy levels. Atoms must __________ energy in order for electrons to move from inner to outer orbits (path
a). And atoms must _________ energy for electrons to move from outer to inner orbits (path
b).
- create, destroy
- destroy, create
- absorb, release
- release, absorb
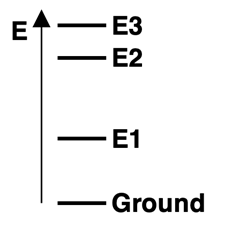
4. Consider the energy level diagram for atoms of Element X, showing the ground state and excited energy levels
E1,
E2, and
E3. Three visible emission lines are observed for this element - red, blue, and violet. Based on the relative energy levels shown in the diagram, match these three colors to the corresponding transitions.
a. E1 State to Ground State:
Red, Blue, or Violet
b. E2 State to Ground State:
Red, Blue, or Violet
c. E3 State to E2 State:
Red, Blue, or Violet