Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 1: Energy and Heat
Part a: Energy
Part a: Energy
Part b: Heat and Temperature
Part c: Chemical Reactions and Energy
Part d: Calorimetry
Part e: Energy and Changes of State
 Defining Energy
Defining Energy
Energy may be one of the more abstract concepts in science. Energy has no mass and no volume. It is not matter or “stuff.” Energy can’t be seen. You only know that it exists because of its effect on objects. When you observe objects warming up or cooling down, speeding up or slowing down, rising up or falling down, you are observing the effects of energy and energy changes.
So, what exactly is energy? In a concrete way, how can energy be defined? Expecting a 1-sentence clarifying answer to such a question is likely an unreasonable expectation. But it is a good starting point; so here it goes:
Energy is the ability to do work or produce heat.
 When you fill up your gas tank at the gas station, you are purchasing energy - the ability to do work or produce heat. The gasoline possesses energy. When it undergoes combustion in the engine, its energy is harnessed to do work and to produce heat. The car starts moving and things gets hot. The ability to do work is expressed by the car moving and the ability to produce heat is expressed by the heating up of the engine parts and the exhaust gases. Doing work is what you desire; you need to drive your car. Producing heat always comes with the territory.
When you fill up your gas tank at the gas station, you are purchasing energy - the ability to do work or produce heat. The gasoline possesses energy. When it undergoes combustion in the engine, its energy is harnessed to do work and to produce heat. The car starts moving and things gets hot. The ability to do work is expressed by the car moving and the ability to produce heat is expressed by the heating up of the engine parts and the exhaust gases. Doing work is what you desire; you need to drive your car. Producing heat always comes with the territory.
Energy in Two Forms
 The two common forms of energy are kinetic energy and potential energy. Kinetic energy is the energy an object possesses due to its motion. An object that is moving possesses kinetic energy. When the octane gasoline in your car is burned, the energy stored in the gasoline is used to propel your car down the roadway. You car acquires kinetic energy. Whether big or small, whether an asteroid or an atom, any object that is moving possesses kinetic energy.
The two common forms of energy are kinetic energy and potential energy. Kinetic energy is the energy an object possesses due to its motion. An object that is moving possesses kinetic energy. When the octane gasoline in your car is burned, the energy stored in the gasoline is used to propel your car down the roadway. You car acquires kinetic energy. Whether big or small, whether an asteroid or an atom, any object that is moving possesses kinetic energy.
 Systems can also possess potential energy. Potential energy is the energy stored in a system due to the interactions of objects within the system. It is often thought of as the energy of position. A mountain climber on a cliff has potential energy relative to the ground due to the gravitational interactions between the climber and the Earth below. As the climber’s height (vertical position) above the Earth increases, so does the potential energy. As we will discuss later in great detail, potential energy is even stored in chemical systems. The chemical potential energy of octane gasoline is the result of the positioning of atoms and the electrical interaction between electrons and protons of its atoms. In a reaction, the atoms are pulled apart and repositioned to form new products. This results in a change in the chemical potential energy. Potential energy is the energy of position stored in a system.
Systems can also possess potential energy. Potential energy is the energy stored in a system due to the interactions of objects within the system. It is often thought of as the energy of position. A mountain climber on a cliff has potential energy relative to the ground due to the gravitational interactions between the climber and the Earth below. As the climber’s height (vertical position) above the Earth increases, so does the potential energy. As we will discuss later in great detail, potential energy is even stored in chemical systems. The chemical potential energy of octane gasoline is the result of the positioning of atoms and the electrical interaction between electrons and protons of its atoms. In a reaction, the atoms are pulled apart and repositioned to form new products. This results in a change in the chemical potential energy. Potential energy is the energy of position stored in a system.
Law of Conservation of Energy
One of the most crosscutting concepts in science is the law of conservation of energy. There are many ways to state the law. Here’s one:
Energy cannot be created nor destroyed. It can only be transformed from one form to another or transferred from one object to another.
The total amount of energy in all its forms and in all objects is unchanging.
 A pendulum bob that swings back and forth is continuously changing its speed and its height. Kinetic energy depends on speed and potential energy depends on height. As the bob swings higher (from B to C or from B to A), it slows down until it reaches its highest position where it has run out of kinetic energy. At that highest position (A or C), its potential energy is a maximum and its kinetic energy is a minimum. As the pendulum bob swings to its lowest position (from A to B or from C to B), its height decreases and its speed increases. At its lowest position (B), its potential energy is a minimum and its kinetic energy is a maximum. But during the entire back-and-forth motion, the total amount of energy is constant. Any losses in kinetic energy are made up for by gains in potential energy … and vice versa. We say that energy is conserved.
A pendulum bob that swings back and forth is continuously changing its speed and its height. Kinetic energy depends on speed and potential energy depends on height. As the bob swings higher (from B to C or from B to A), it slows down until it reaches its highest position where it has run out of kinetic energy. At that highest position (A or C), its potential energy is a maximum and its kinetic energy is a minimum. As the pendulum bob swings to its lowest position (from A to B or from C to B), its height decreases and its speed increases. At its lowest position (B), its potential energy is a minimum and its kinetic energy is a maximum. But during the entire back-and-forth motion, the total amount of energy is constant. Any losses in kinetic energy are made up for by gains in potential energy … and vice versa. We say that energy is conserved.
Now if you’ve watched a real pendulum (not the simulated one shown above), you know that the height to which the pendulum swings becomes less and less on each consecutive cycle. The vibrations eventually cease or die out. Doesn’t that violate the law of conservation of energy? That’s a great question, whether you were thinking it or not. And the answer is that it doesn’t. The law doesn’t state that an object conserves its own energy. The law states that the sum of all the energy in all the objects is conserved. The pendulum interacts with the surrounding air as it swings through the air. Collisions with air molecules cause the air to start moving. We refer to this as air resistance. The air molecules gain kinetic energy while the pendulum loses its total energy.
The System and Surroundings
Energy conservation often requires that we distinguish between the system and the surroundings. The system might be the pendulum or the collection of chemical reactants and products. The system is the part of the universe that you are studying. The surroundings is the rest of the universe. The law of conservation of energy claims that the total energy of the system and the surroundings is constant. If the system loses energy, then the surroundings gain the same amount of energy. If the system gains energy, then the surroundings must have lost the same amount of energy. But the total amount of energy in the system plus surroundings remains the same.
 In the case of the pendulum, the swinging bob was the system - the part of the universe we were studying. The surroundings was the air and the rest of the universe. As the pendulum bob gradually lost energy, the surroundings gradually gained energy. Energy can always transfer between a system and the surroundings as long as the sum of the total energy of the system plus the surroundings remains constant. We often represent these changes in energy by a system diagram. An arrow is used to indicate whether energy exits the system to the surroundings or enters the system from the surroundings.
In the case of the pendulum, the swinging bob was the system - the part of the universe we were studying. The surroundings was the air and the rest of the universe. As the pendulum bob gradually lost energy, the surroundings gradually gained energy. Energy can always transfer between a system and the surroundings as long as the sum of the total energy of the system plus the surroundings remains constant. We often represent these changes in energy by a system diagram. An arrow is used to indicate whether energy exits the system to the surroundings or enters the system from the surroundings.
In chemistry, it is convenient to think of the collection of reactants and products as the system. In the example of filling up your car at the gas tank, the octane gasoline, the oxygen it reacts with, and the CO2 and H2O products would be regarded as the system. There is a lot of energy that can be acquired by the reaction of 12 gallons of octane gasoline. It has the ability to do work on your car many times over for the next several days. But eventually your car ends up back at the same gas station, out of gasoline and with no kinetic energy or potential energy. How is that conservation of energy? What happened to the “energy stored in those 12 gallons”? It escaped to the surroundings as heat. The engine warmed up  and then released its heat to the surroundings. The brake linings warmed up and released their heat to the surroundings. The hot exhaust gases released its heat to the surroundings. The car tires warmed up the road surfaces, releasing heat to the surroundings. Much of the octane energy did useful work to get your car town. Remember: producing heat always comes with the territory.
and then released its heat to the surroundings. The brake linings warmed up and released their heat to the surroundings. The hot exhaust gases released its heat to the surroundings. The car tires warmed up the road surfaces, releasing heat to the surroundings. Much of the octane energy did useful work to get your car town. Remember: producing heat always comes with the territory.
Keeping Track of Energy
It is often easier and more practical to keep track of energy than it is to define it. It is a primary goal of science (and science students) to keep track of energy - its form, its location (in the system or outside it), and how it changes over the course of time. In this chapter we will discuss how chemists analyze chemical systems to determine how energy changes form and the impact that it has upon the surroundings. In the next part of Lesson 1, we will learn about an important part of the puzzle - the relationship between heat and energy.
Before You Leave
- The Check Your Understanding section below include questions with answers and explanations. It provides a great chance to self-assess your understanding.
Check Your Understanding
Use the following questions to assess your understanding. Tap the
Check Answer buttons when ready.
1. Identify the following as being an example of kinetic energy or potential energy.
- A car is moving down the road.
- Having reached the top of the mountain, the car parks in the park’s visitors center.
- The drive turns the ignition and revs up the engine. Hot exhaust gases emerge from the tail pipe.
- The gas tank has been filled and the octane gasoline is just waiting to be used.
- After the car has skidded to a stop, the brake linings become very hot.
2. In your own words, create a 1-2 sentence explanation of what the word
conserved means.
3. So the examples given include energy crossing the system boundary and entering the surroundings. Can energy ever enter a system from the surroundings?
4. Consider the following scenario:
- A pendulum bob starts at rest from its pulled-back position.
- After being released, it swings to its lowest position.
- At its lowest position, it collides with a box of equal mass that is at rest on the floor. The pendulum bob stops and the box is set into motion.
- After sliding a couple of meters across the floor, the box finally stops due to the friction with the floor.
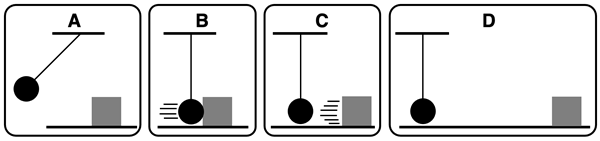
Track the energy of the system and surroundings by completing the following paragraph.
The system includes the pendulum bob and the box. In state A, the pendulum bob possesses ____________ (kinetic, potential, no) energy. When the bob reaches its lowest position (B), the bob possesses ____________ (kinetic, potential, no) energy. After colliding with the box (C), the bob possesses ____________ (kinetic, potential, no) energy and the box possesses ____________ (kinetic, potential, no) energy. After the box finally stops (D), the bob possesses ____________ (kinetic, potential, no) energy and the box possesses ____________ (kinetic, potential, no) energy. This indicates that _______________________________________ (energy is not conserved, the energy of the system transferred to the surroundings). The result of this is that ______________________________ (the laws of science can’t be trusted, the floor warmed up and sound energy resulted from the bob-box collision).